Abstract
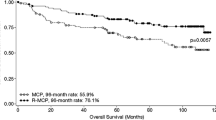
Follicular lymphoma is a lymphoid malignancy commonly showing slow progression which makes the treatment of the disease challenging. Rituximab monotherapy and rituximab added to standard chemotherapy has been proven to increase survival among patients with advanced stage of the disease. However, the benefit of a rituximab maintenance therapy after induction was still unclear at the time of the initiation of this study. HUSOM was a phase III open-label, single-arm, multi-centre study aimed to assess the efficacy and the safety of the 12 cycles of rituximab (375 mg/m2 every 8 weeks) maintenance therapy in patients had already presented partial or complete response to R-CVP or R-CHOP. Efficacy endpoints such as event-free survival and overall survival were estimated. Adverse events were recorded during the entire course of the study. A total number of 124 patients were enrolled by 15 Hungarian study sites. Out of these, 86 patients received 12 cycles of rituximab and 69 patients completed the 3-year follow-up phase as well. The probabilities of the event free survival and progression at 4.3 years were estimated to be 70.3% and 74.4%, respectively. The overall and the disease free survival at 4 years were estimated to be 90.7% and 87.9%, respectively. A total number of 85 adverse events were reported during the study out of which 5 AEs were considered to be related to the administration of rituximab. Analyses of the efficacy variables have revealed comparable results to those reported by controlled clinical trials (EORTC 20981, PRIMA) conducted in parallel with the HUSOM study.



Similar content being viewed by others
References
Greenhalgh J, Bagust A, Boland A et al (2013) Rituximab for the first-line maintenance treatment of follicular non-Hodgkin's lymphoma: a NICE single technology appraisal. PharmacoEconomics 31(5):403–413
Gallagher CJ, Gregory WM, Jones AE et al (1986) Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol 4(10):1470–1480
Piro LD, White CA, Grillo-López AJ et al (1990) Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol 10:655–661
Tedder TF, Engel P (1994) CD20: a regulator of cell-cycle progression of B lymphocytes. Immunol Today 15:450–454
Reff ME, Carner K, Chambers KS (1994) Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83:435–445
Maloney D, Smith B, Appelbaum F (1996) The anti-tumor effect of monoclonal anti-CD20 antibody (MAB) therapy includes direct anti-proliferative activity and induction of apopthosis in CD20 positive non-Hodgkin’s lymphoma (NHL) cell lines. Blood 88:637a
Maloney DG, Liles TM, Czerwinski DK et al (1994) Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 84:2457–2466
McLaughlin P, Grillo-López AJ, Link BK et al (1998) Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 16:2825–2833
Czuczman MS, Grillo-Lopez AJ, White CA et al (1999) Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol 17:268–276
Czuczman MS, Fallon A, Mohr A et al (2001) Phase II study of rituximab plus fludarabine in patients (pts) with low-grade lymphoma (LGL): final report. ASH 2001 Abstr no.: 2518
Hiddemann W, Dreyling MH, Forstpointner R et al (2003) Combined Immuno-chemotherapy (R-CHOP) significantly improves time to treatment failure in first line therapy of follicular lymphoma results of a prospective randomized trial of the German low grade lymphoma study group (GLSG). ASH Abstr no.: 352
Marcus R, Imrie K, Belch A et al (2003) An international multi-centre, randomized, open-label, phase III trial comparing rituximab added to CVP chemotherapy to CVP chemotherapy alone in untreated stage III/IV follicular non-Hodgkins lymphoma. ASH Abstr no.: 87
Dreyling MH, Forstpointner R, Repp R et al (2003) Combined Immuno-chemotherapy (R-FCM) results in superior remission and survival rates in recurrent follicular and mantle cell lymphoma final results of a prospective randomized trial of the German low grade lymphoma study group (GLSG). ASH Abstr. No.: 351
McLaughlin P, Rodriguez MA, Hagemeister FB et al (2003) Stage IV indolent lymphoma: a randomized study of concurrent vs. sequential use of FND chemotherapy (fludarabine, mitoxantrone, dexamethasone) and rituximab (R) monoclonal antibody therapy, with interferon maintenance. Proc am Soc Clin Oncol 22:564
Vitolo U, Boccomini C, Ladetto M et al (2002) High clinical and molecular response rate in elderly patients with advanced stage follicular lymhoma treated at diagnosis with a brief chemo-immunotherapy FND + rituximab. ASH Abstr no.: 1392
Gregory SA, Venugopal P, Adler S et al (2003) A phase II study of fludarabine phosphate and mitoxantrone followed by anti-CD20 monoclonal antibody in the treatment of patients with newly diagnosed, advanced low-grade non-Hodgkin′s lymphoma (LGNHL): interim results ASH Abstr no.:1499
Czuczman MS, Koryzna A, Mohr A et al (2005) Rituximab in combination with fludarabine chemotherapy in low-grade or follicular lymphoma. J Clin Oncol 2005 23(4):694–704
Maloney DG, Grillo-Lopez AJ, Bodkin DJ et al (1997) IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol 15:3266–3274
Maloney DG, Grillo-Lopez AJ, White CA et al (1997) IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 90:2188–2195
Davis TA, Grillo-Lopez AJ, White CA et al (2000) Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol 18:3135–3143
Hainsworth JD, Litchy S, Morrissey L et al (2003) Rituximab as first-line and maintenance therapy for indolent non-Hodgkin′s lymphoma (NHL): long-term follow-up of a minnie pearl cancer research network phase II trial. ASH Abstr no.: 1496
Ghielmini M, Schmitz SFH, Cogliatti SB et al (2004) Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood 103(12):4416–4423
Hochster H, Weller E, Gascoyne RD et al (2009) Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: results of the randomized phase III ECOG1496 study. J Clin Oncol 2009 27(10):1607–1614
van Oers MH, Klasa R, Marcus RE et al (2006) Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial. Blood 108(10):3295–3301
Salles GL, Seymour JF, Offner F et al (2011) Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet 377(9759):42–51
van Oers MH, Van Glabbeke M, Giurgea L et al (2010) Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol 28(17):2853–2858
Salles GL, Seymour JF, Feugier P et al (2013) Updated 6 year follow-up of the PRIMA study confirms the benefit of 2-year rituximab maintenance in follicular lymphoma patients responding to frontline Immunochemotherapy. Blood 122(21):509
Dreyling M, Ghielmini M, Rule S et al (2016) ESMO guidelines committee. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27(suppl 5):v83–v90
Acknowledgments
We would like to thank the patients, their families, the nurses and the investigators who are participating in this study. Funding for the HUSOM study was provided by F. Hoffmann-La Roche Ltd., Basel, Switzerland. Support for third-party medical writing for this article by Accepther Ltd. was provided by Roche Hungary Ltd., Budapest.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was carried out in accordance with the rules of Good Clinical Practice (GCP) as well as with the basic principles of “Declaration of Helsinki (Fortaleza, 2013)” and was approved by the National Institute of Pharmacy (OGYI, Hungary) and by the Clinical Pharmacology Ethical Committee of the Medical Science Council (ETT-KFEB, Hungary).
Conflict of Interest
TS: honoraria, travel reimbursement; AI: advisory role; LS: honoraria; ZG: travel reimbursement; TM: advisory role; JD: advisory role; PD: honoraria; ZB: advisory role; remaining authors have declared no conflicts of interest.
Additional information
Antal Tóth died before publication of this work was completed.
Rights and permissions
About this article
Cite this article
Schneider, T., Rosta, A., Losonczy, H. et al. Efficacy and Tolerability of a 2-Year Rituximab Maintenance Therapy in Patients with Advanced Follicular Lymphoma after Induction of Response with Rituximab-Containing First Line-Regimens (HUSOM Study). Pathol. Oncol. Res. 24, 199–205 (2018). https://doi.org/10.1007/s12253-017-0234-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0234-2