Abstract
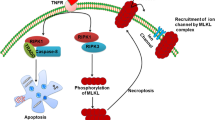
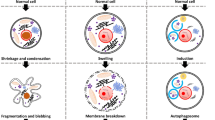
Programmed cell death is a key component of tissue homeostasis, normal development and wide variety of diseases. Conventional view refers to programmed cell death form as caspase-mediated apoptosis while necrosis is considered as an accidental and unwanted cell demise, carried out in a non-regulated manner and caused by extreme conditions. However, accumulating evidences indicate that necrotic cell death can also be a regulated process. The term necroptosis has been introduced to describe a cell death receptor-induced, caspase-independent, highly regulated type of programmed cell death process with morphological resemblance of necrosis. Necroptosis recently has been found to contribute to a wide range of pathologic cell death forms including ischemic brain injury, neurodegenerative diseases and viral infection, therefore a better understanding of the necroptotic signaling machinery has clinical relevance.
Similar content being viewed by others
Abbreviations
- PCD:
-
Programmed cell death
- LC3:
-
Microtubule-associated protein light chain 3
- CICD:
-
Caspase-independent cell death
- FasL:
-
(also known CD95L) Tumor necrosis factor ligand superfamily member 6
- Fas receptor:
-
(also known CD95 receptor) Tumor necrosis factor receptor superfamily member 6
- TNFα:
-
Tumor necrosis factor-alpha, tumor necrosis factor
- RIPK1:
-
(also known RIP1) Receptor-interacting protein kinase 1, receptor-interacting serine/threonine-protein kinase 1
- TRAIL:
-
(also known Apo-2L) Tumor necrosis factor-related apoptosis-inducing ligand, tumor necrosis factor ligand superfamily member 10
- Nec-1:
-
Necrostatin-1
- TNF-R1:
-
Tumor necrosis factor receptor 1, tumor necrosis factor receptor superfamily member 1A
- TNF-R2:
-
Tumor necrosis factor receptor 2, tumor necrosis factor receptor superfamily member 1B
- TRAIL-R1:
-
Tumor necrosis factor-related apoptosis-inducing ligand receptor 1, tumor necrosis factor receptor superfamily member 10A
- TRAIL-R2:
-
Tumor necrosis factor-related apoptosis-inducing ligand receptor 2, tumor necrosis factor receptor superfamily member 10B
- TRADD:
-
TNFα receptor-associated death domain protein, tumor necrosis factor receptor type 1-associated death domain protein
- TRAF2:
-
TNFα receptor-associated factor 2, TNF receptor-associated factor 2
- TRAF5:
-
TNFα receptor-associated factor 5, TNF receptor-associated factor 5
- IAP-1:
-
Inhibitor of apoptosis protein 1, baculoviral IAP repeat-containing protein 3
- IAP-2:
-
Inhibitor of apoptosis protein 2, baculoviral IAP repeat-containing protein 2
- NEMO:
-
NF-kappa-B essential modulator
- IKK:
-
IκB kinase
- TAK1:
-
Transforming growth factor β-activated kinase 1
- TAB2:
-
TAK1 binding protein 2
- NFkB:
-
Nuclear factor NF-kappa-B
- FADD:
-
Fas-associated death domain protein
- RIPK3:
-
(also known RIP3) Receptor-interacting protein kinase 3, receptor-interacting serine/threonine-protein kinase 3
- DISC:
-
Death-inducing signaling complex
- CYLD:
-
Ubiquitin carboxyl-terminal hydrolase
- RHIM:
-
RIP homotypic interaction motif
- PYGL:
-
Glycogen phosphorylase
- GLUL:
-
Glutamine synthetase
- GLUD1:
-
Glutamate dehydrogenase 1
- ROS:
-
Reactive oxygen species
- NOX-1:
-
NADPH oxidase 1
- BHA:
-
Butylated hydroxyanisole
- ANT:
-
Adenine nucleotide translocator, ADP/ATP translocase
- CYPD:
-
Cyclophilin D
- mPTP:
-
Mitochondrial permeability transition pore
- ATP:
-
Adenosine triphosphate
- APAF1:
-
Apoptotic protease-activating factor 1
- BAK:
-
Bcl-2 homologous antagonist/killer
- BAX:
-
Bcl-2-like protein 4
- DIF-1:
-
Differentiation-inducing factor
- atg1:
-
Autophagy related 1 homolog gene
- zVAD.fmk:
-
Carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]- fluoromethylketone
- NK cell:
-
Natural killer cell
- CCI:
-
Controlled cortical impact
- TBI:
-
Traumatic brain injury
- NMDA:
-
N-Methyl-D-aspartate
- LDH:
-
Lactate dehydrogenase
- GSH:
-
Glutathione
- AIF:
-
Apoptosis-inducing factor
- PAR:
-
poly(ADP-ribose)chain
- AA:
-
Arachidonic acid
- LOX:
-
Lypoxigenase
- JNK:
-
c-Jun N-terminal kinase 1, mitogen-activated protein kinase 8
- NO:
-
Nitric oxide
- MnSOD:
-
Superoxide dismutase [Mn]
- ACAT:
-
Acyl-CoA:cholesterol acyltransferase
- CHOP:
-
C/EBP homologous protein
- cFLIP:
-
Cellular FLICE-like inhibitory protein, CASP8 and FADD-like apoptosis regulator
- MDR-ABC:
-
Multi-drug resistant ATP binding cassette
- AML:
-
Acute myeloid leukemia
- GM-CSF:
-
Granulocyte-macrophage colony stimulating factor
- CAD:
-
Caspase-activated DNAse
- ICAD:
-
Inhibitor of CAD
- DED:
-
Death effector domain
- cFLIP:
-
Cellular FLICE-inhibitory protein
- ROCK1:
-
Rho-associated protein kinase 1
- RAIDD:
-
Receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a DD
References
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
Metzstein MM, Stanfield GM, Horvitz HR (1998) Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet 14(10):410–416
Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P et al (2005) Classification of cell death: recommendations of the nomenclature committee on cell death. Cell Death Differ 12(suppl 2):1463–1467
Déas O, Dumont C, MacFarlane M, Rouleau M, Hebib C, Harper F et al (1998) Caspase-independent cell death induced by anti-CD2 or staurosporine in activated human peripheral T lymphocytes. J Immunol 161(7):3375–3383
Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol 146(1):3–15
Lemasters JJ (1999) V. Necrapoptosis and the mitochondrial permeability transition: shared pathways to necrosis and apoptosis. Am J Physiol 276(1):G1–6
Li M, Beg AA (2000) Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol 74(16):7470–7477
Borst P, Rottenberg S (2004) Cancer cell death by programmed necrosis? Drug Resist Updat 7(6):321–324
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1(2):112–119
Vercammen D, Brouckaert G, Denecker G, Van De Craen M, Declercq W, Fiers W et al (1998) Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med 188(5):919–930
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S et al (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489–495
Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S (1998) Caspase-independent cell killing by Fas-associated protein with death domain. J Cell Biol 143(5):1353–1360
Laster SM, Wood JG, Gooding LR (1988) Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol 141(8):2629–2634
Schulze-Osthoff K, Krammer PH, Dröge W (1994) Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J 13(19):4587–4596
Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV (1996) TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4(4):387–396
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X et al (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4(5):313–321
Yuan J (2009) Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis 14(4):469–477
Neumar RW (2000) Molecular mechanisms of ischemic neuronal injury. Ann Emerg Med 36(5):483–506
McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S (2004) Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 286(5 55–5):H1923–H1935
Zhang X, Chen Y, Jenkins LW, Kochanek PM, Clark RSB (2005) Bench-to-bedside review: apoptosis/programmed cell death triggered by traumatic brain injury. Crit Care 9(1):66–75
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W et al (1998) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187(9):1477–1485
Chan FKM, Shisler J, Bixby JG, Felices M, Zheng L, Appel M et al (2003) A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278(51):51613–51621
Andera L (2009) Signaling activated by the death receptors of the TNFR family. Biomed Pap 153(3):173–180
Micheau O, Tschopp J (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114(2):181–190
Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ (2006) Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell 22(2):245–257
Chen ZJ (2005) Ubiquitin signalling in the NF-kB pathway. Nat Cell Biol 7(8):758–765
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11(10):700–714
Cho Y, Challa S, Moquin D, Genga R, Ray TD, Guildford M et al (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137(6):1112–1123
Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N et al (2007) Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 19(10):2056–2067
Jin Z, El-Deiry WS (2006) Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol Cell Biol 26(21):8136–8148
Harper N, Hughes M, MacFarlane M, Cohen GM (2003) Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor I signaling complex during tumor necrosis factor-induced apoptosis. J Biol Chem 278(28):25534–25541
Morgan MJ, Kim YS, Liu ZG (2009) Membrane-bound Fas ligand requires RIP1 for efficient activation of caspase-8 within the death-inducing signaling complex. J Immunol 183(5):3278–3284
Lavrik IN. (2011) Regulation of death receptor-induced apoptosis induced via CD95/Fas and other death receptors. Mol Biol 45(1):150–155
Gonzalvez F, Ashkenazi A. (2010) New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 29(34):4752–4765
Sakahira H, Enari M, Nagata S (1998) Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391(6662):96–99
Lüthi AU, Martin SJ (2007) The CASBAH: a searchable database of caspase substrates. Cell Death Diff 14(4):641–650
Bulat N, Widmann C (2009) Caspase substrates and neurodegenerative diseases. Brain Res Bull 80(4–5):251–267
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V et al (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388(6638):190–195
Oosten ASV, Navis G, Stegeman CA, Joles JA, Klok PA, Kuipers F et al (2001) Increased expression of cFLIPL in colonic adenocarcinoma. J Pathol 194(1):15–19
Tepper CG, Seldin MF (1999) Modulation of caspase-8 and FLICE-inhibitory protein expression as a potential mechanism of Epstein-Barr virus tumorigenesis in Burkitt’s lymphoma. Blood 94(5):1727–1737
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ et al (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135(7):1311–1323
He S, Wang L, Miao L, Wang T, Du F, Zhao L et al (2009) Receptor interacting protein Kinase-3 determines cellular necrotic response to TNF-α. Cell 137(6):1100–1111
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC et al (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938):332–336
Vanden Berghe T, Declercq W, Vandenabeele P (2007) NADPH Oxidases: new players in TNF-induced necrotic cell death. Mol Cell 26(6):769–771
Kim YS, Morgan MJ, Choksi S, Liu ZG (2007) TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 26(5):675–687
Temkin V, Huang Q, Liu H, Osada H, Pope RM (2006) Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol 26(6):2215–2225
Berghe TV, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N et al. (2010) Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17(6):922–930
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S et al (2004) Regulation of an ATG7-beclin 1 program of autophaglic cell death by caspase-8. Science 304(5676):1500–1502
Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK et al. (2010) Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest 120(4):1310–1323
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R et al (1998) Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94(6):739–750
Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA et al (2000) The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 6(6):1389–1399
Lindsten T, Thompson CB (2006) Cell death in the absence of Bax and Bak. Cell Death Differ 13(8):1272–1276
Laporte C, Kosta A, Klein G, Aubry L, Lam D, Tresse E et al (2007) A necrotic cell death model in a protist. Cell Death Differ 14(2):266–274
Barkla DH, Gibson PR (1999) The fate of epithelial cells in the human large intestine. Pathology 31(3):230–238
Emons J, Chagin AS, Hultenby K, Zhivotovsky B, Wit JM, Karperien M et al (2009) Epiphyseal fusion in the human growth plate does not involve classical apoptosis. Pediatr Res 66(6):654–659
Kumar A, Rothman J (2007) Cell death: hook, line and linker. Curr Biol 17(8):R286–R289
Abraham MC, Lu Y, Shaham S (2007) A morphologically conserved nonapoptotic program promotes linker cell death in Caenorhabditis elegans. Dev Cell 12(1):73–86
Yuan J, Kroemer G. (2010) Alternative cell death mechanisms in development and beyond. Genes Dev 24(23):2592–2602
McCall K. (2010) Genetic control of necrosis-another type of programmed cell death. Curr Opin Cell Biol 22(6):882–888
Zong WX, Thompson CB (2006) Necrotic death as a cell fate. Genes Dev 20(1):1–15
Golstein P, Kroemer G (2005) Redundant cell death mechanisms as relics and backups. Cell Death Differ 12(suppl 2):1490–1496
Benedict CA, Norris PS, Ware CF (2002) To kill or be killed: viral evasion of apoptosis. Nat Immunol 3(11):1013–1018
Berglund AC, Sjolund E, Östlund G, Sonnhammer ELL (2008) InParanoid 6: eukaryotic ortholog clusters with inparalogs. Nucleic Acids Res 36(suppl 1):D263–D266
Mareninova OA, Sung KF, Hong P, Lugea A, Pandol SJ, Gukovsky I et al (2006) Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem 281(6):3370–3381
West T, Atzeva M, Holtzman DM (2006) Caspase-3 deficiency during development increases vulnerability to hypoxic-ischemic injury through caspase-3-independent pathways. Neurobiol Dis 22(3):523–537
Stoll G, Jander S, Schroeter M (2002) Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol 513:87–113
Zhang M, Chen L (2008) Status of cytokines in ischemia reperfusion induced heart injury. Cardiovasc Hematol Disord Drug Targets 8(3):161–172
You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD et al (2008) Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 28(9):1564–1573
Li Y, Yang X, Ma C, Qiao J, Zhang C (2008) Necroptosis contributes to the NMDA-induced excitotoxicity in rat’s cultured cortical neurons. Neurosci Lett 447(2–3):120–123
Tan S, Schubert D, Maher P (2001) Oxytosis: a novel form of programmed cell death. Curr Top Med Chem 1(6):497–506
Xu X, Chua CC, Kong J, Kostrzewa RM, Kumaraguru U, Hamdy RC et al (2007) Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem 103(5):2004–2014
Cregan SP, Dawson VL, Slack RS (2004) Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23(16 REV. ISS. 2):2785–2796
Kim S, Dayani L, Rosenberg PA, Li J. (2010) RIP1 kinase mediates arachidonic acid-induced oxidative death of oligodendrocyte precursors. Int J Physiol Pathophysiol Pharmacol 2(2):137–147
Davis CW, Hawkins BJ, Ramasamy S, Irrinki KM, Cameron BA, Islam K et al. (2010) Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic Biol Med 48(2):306–317
Smith CCT, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM (2007) Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther 21(4):227–233
Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CCT (2007) The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther 21(6):467–469
Bao L, Li Y, Deng SX, Landry D, Tabas I (2006) Sitosterol-containing lipoproteins trigger free sterol-induced caspase-independent death in ACAT-competent macrophages. J Biol Chem 281(44):33635–33649
Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y et al (2007) Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther 6(5):1641–1649
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Hu W, Kavanagh JJ (2003) Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 4(12):721–729
Li QX, Yu DH, Liu G, Ke N, McKelvy J, Wong-Staal F (2008) Selective anticancer strategies via intervention of the death pathways relevant to cell transformation. Cell Death Differ 15(8):1197–1210
Horita H, Frankel AE, Thorburn A (2008) Acute myeloid leukemia-targeted toxin activates both apoptotic and necroptotic death mechanisms. PLoS ONE 3(12):e3909
Tinel A, Tschopp J (2004) The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304(5672):843–846
Janssens S, Tinel A, Lippens S, Tschopp J (2005) PIDD mediates NF-kB activation in response to DNA damage. Cell 123(6):1079–1092
Kim IR, Murakami K, Chen NJ, Saibil SD, Matysiak-Zablocki E, Elford AR et al (2009) DNA damage- and stress-induced apoptosis occurs independently of PIDD. Apoptosis 14(9):1039–1049
Tu HC, Ren D, Wang GX, Chen DY, Westergard TD, Kim H et al (2009) The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proc Natl Acad Sci USA 106(4):1093–1098
Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB (2004) Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev 18(11):1272–1282
Hanahan D, Weinberg RA. (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Grivennikov SI, Greten FR, Karin M. (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899
Acknowledgements
This work was supported by grant ETT 016/2008 for PIB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dunai, Z., Bauer, P.I. & Mihalik, R. Necroptosis: Biochemical, Physiological and Pathological Aspects. Pathol. Oncol. Res. 17, 791–800 (2011). https://doi.org/10.1007/s12253-011-9433-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-011-9433-4