Abstract
Purpose
The objective of the current research is to formulate a nanoemulsion (NE) of nimodipine (ND) to improve its oral bioavailability and stability by adopting a scientific and systematic quality-by-design (QbD) approach.
Methods
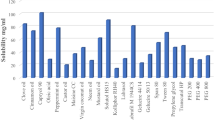
Triacetin (oil), Cremophor RH40 (surfactant), and PEG600 (co-surfactant) were selected to formulate NE. Factors affecting critical quality attributes (CQA) were screened by Taguchi design followed by Box-Behnken design (BBD) enabled optimization. The optimized NE was further lyophilized with trehalose (cryoprotectant) to improve the storage stability.
Results
Optimized NE (F4), and its lyophilized NE showed globule size less than 200 nm with more than 75% drug diffusion within 30 min. Both NE (F4) and its lyophilized NE exhibited a fivefold increase in area under the curve in comparison to an aqueous dispersion of ND. Lyophilized F4 showed better stability due to the conversion of NE into solid form by lyophilization.
Conclusion
Lyophilized NE of ND can be prepared for improved oral bioavailability and stability. The above approach is simple, cost-effective, and scalable to manufacture on a commercial scale.
Graphical Abstract












Similar content being viewed by others
Data Availability
All data pertaining to this research will be made available.
References
Inzitari DPA. Calcium channel blockers and stroke. Aging Clin Exp Res. 2005;17:16–30.
Fu Q, Sun J, Ai X, et al. Nimodipine nanocrystals for oral bioavailability improvement: role of mesenteric lymph transport in the oral absorption. Int J Pharm. 2013;448:290–7.
Luo JW, Zhang ZR, Gong T, et al. One-step self-assembled nanomicelles for improving the oral bioavailability of nimodipine. Int J Nanomedicine. 2016;11:1051–65.
Azeem A, Rizwan M, Ahmad FJ, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10:69–76.
Cj P, Nl T, Wn C. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–48.
Chhabra G, Chuttani K, Mishra AK, et al. Design and development of nanoemulsion drug delivery system of amlodipine besilate for improvement of oral bioavailability. Drug Dev Ind Pharm. 2011;37:907–16.
Veerareddy PR, Poluri K, Sistla R. Formulation development and comparative pharmacokinetic evaluation of felodipine nanoemulsions in SD rats. Am J PharmTech Res. 2012;2:931–945.
Khani S, Keyhanfar F, Amani A. Design and evaluation of oral nanoemulsion drug delivery system of mebudipine. Drug Deliv. 2016;23:2035–43.
Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–7.
Shekhawat P, Pokharkar V. Risk assessment and QbD based optimization of an Eprosartan mesylate nanosuspension: in-vitro characterization, PAMPA and in-vivo assessment. Int J Pharm. 2019. https://doi.org/10.1016/j.ijpharm.2019.06.006.
Panigrahi KC, Jena J, Jena GK, et al. QBD-based systematic development of Bosentan SNEDDS: formulation, characterization and pharmacokinetic assessment. J Drug Deliv Sci Technol. 2018;47:31–42.
Sun Y, Rui Y, Wenliang Z, et al. Nimodipine semi-solid capsules containing solid dispersion for improving dissolution. Int J Pharm. 2008;359:144–9.
Chudasama A, Patel V, Nivsarkar M, et al. Role of lipid-based excipients and their composition on the bioavailability of antiretroviral self-emulsifying formulations. Drug Deliv. 2015;22:531–40.
Kassem AA, Mohsen AM, Ahmed RS, et al. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: Design, optimization, in vitro and in vivo evaluation. J Mol Liq. 2016;218:219–32.
Routray SB, Patra CN, Raju R, et al. Lyophilized SLN of Cinnacalcet HCl: BBD enabled optimization, characterization and pharmacokinetic study. Drug Dev Ind Pharm. 2020;46:1080–91.
Czajkowska-Kos̈nik A, Szekalska M, Amelian A, et al. Development and evaluation of liquid and solid self-emulsifying drug delivery systems for atorvastatin. Molecules. 2015;20. https://doi.org/10.3390/molecules201219745.
Inugala S, Eedara BB, Sunkavalli S, et al. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) of darunavir for improved dissolution and oral bioavailability: In vitro and in vivo evaluation. Eur J Pharm Sci. 2015;74:1–10.
Sharma P, Singh SK, Pandey NK, et al. Impact of solid carriers and spray drying on pre/post-compression properties, dissolution rate and bioavailability of solid self-nanoemulsifying drug delivery system loaded with simvastatin. Powder Technol. 2018;338:836–46.
Gurumukhi VC, Bari SB. Development of ritonavir-loaded nanostructured lipid carriers employing quality by design (QbD) as a tool: characterizations, permeability, and bioavailability studies. Drug Deliv Transl Res. 2021;2021:1–21.
Arsiccio A, Pisano R. Application of the quality by design approach to the freezing step of freeze-drying: building the design space. J Pharm Sci. 2018. https://doi.org/10.1016/j.xphs.2018.02.003.
Laxmi M, Bhardwaj A, Mehta S, et al. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif Cells, Nanomedicine Biotechnol. 2015;43:334–44.
Srinivas NSK, Verma R, Kulyadi GP, et al. A quality by design approach on polymeric nanocarrier delivery of gefitinib: formulation, in vitro, and in vivo characterization. Int J Nanomedicine. 2017;12:15–28.
Parmar N, Singla N, Amin S, et al. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surfaces B Biointerfaces. 2011;86:327–38.
Ujhelyi Z, Vecsernyés M, Fehér P, et al. Physico-chemical characterization of self-emulsifying drug delivery systems. Drug Discov Today Technol. 2018;27:81–6.
Xue X, Cao M, Ren L, et al. Preparation and optimization of rivaroxaban by self-nanoemulsifying drug delivery system (SNEDDS) for enhanced oral bioavailability and no food effect. AAPS PharmSciTech. 2018. https://doi.org/10.1208/s12249-018-0991-6.
AboulFotouh K, Allam AA, El-Badry M, et al. Development and in vitro/in vivo performance of self-nanoemulsifying drug delivery systems loaded with candesartan cilexetil. Eur J Pharm Sci. 2017;109:503–13.
Park SJ, Choo GH, Hwang SJ, et al. Quality by design: Screening of critical variables and formulation optimization of Eudragit e nanoparticles containing dutasteride. Arch Pharm Res. 2013;36:593–601.
Yasir M, Sara UVS, Chauhan I, et al. Solid lipid nanoparticles for nose to brain delivery of donepezil: formulation, optimization by Box-Behnken design, in vitro and in vivo evaluation. Artif Cells, Nanomedicine Biotechnol. 2018;46:1838–51.
Gade MM, Hurkadale PJ. Formulation and evaluation of self-emulsifying orlistat tablet to enhance drug release and in vivo performance: factorial design approach. Drug Deliv Transl Res. 2016. https://doi.org/10.1007/s13346-016-0289-8.
Patel P, Pailla SR, Rangaraj N, et al. Quality by design approach for developing lipid-based nanoformulations of gliclazide to improve oral bioavailability and anti-diabetic activity. AAPS PharmSciTech. 2019. https://doi.org/10.1208/s12249-018-1214-x.
Zhang JQ, Liu J, Li XL, et al. Preparation and characterization of solid lipid nanoparticles containing silibinin. Drug Deliv. 2007;14:381–7.
Kasongo KW, Pardeike J, Müller RH, et al. Selection and characterization of suitable lipid excipients for use in the manufacture of didanosine-loaded solid lipid nanoparticles and nanostructured lipid carriers. J Pharm Sci. 2011;100:5185–96.
Aa K, Vb P. Design and evaluation of self-emulsifying drug delivery systems (SEDDS) of nimodipine. AAPS PharmSciTech. 2008;9:191–6.
Panigrahi KC, Patra CN, Rao MEB. Quality by design enabled development of oral self-nanoemulsifying drug delivery system of a novel calcimimetic cinacalcet HCl using a porous carrier: in vitro and in vivo characterisation. AAPS PharmSciTech. 2019. https://doi.org/10.1208/s12249-019-1411-2.
Jena GK, Patra CN, Panigrahi KC, et al. QbD enabled optimization of solvent shifting method for fabrication of PLGA-based nanoparticles for promising delivery of capecitabine for antitumor activity. Drug Deliv Transl Res. 2021. https://doi.org/10.1007/S13346-021-01042-0.
Quality Guidelines : ICH. 2018. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html. Accessed 26 Dec 2018.
Tartaro G, Mateos H, Schirone D, et al. Microemulsion microstructure(s): a tutorial review. Nanomater (Basel, Switzerland). 2020;10:1–40.
Gupta S, Kesarla R, Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013;2013:1–16.
Acknowledgements
The authors would like thank Dr. Sukant Tripathy, Professor, Berhampur University, India for providing facility for XRD study.
Author information
Authors and Affiliations
Contributions
All authors contributed for this research manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This research is approved by Institutional Anima Ethical Committee (IAEC) of Roland Institute of Pharmaceutical Sciences, Berhampur, with the following approval number 103 (Regd.No.926/PO/ac/06/CPCSEA).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patra, C.N., Mishra, A., Jena, G.K. et al. QbD Enabled Formulation Development of Nanoemulsion of Nimodipine for Improved Biopharmaceutical Performance. J Pharm Innov 18, 1279–1297 (2023). https://doi.org/10.1007/s12247-023-09714-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-023-09714-9