Abstract
Purpose
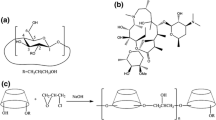
The aim of this study was to prepare tosufloxacin tosylate (TFLX) and hydroxypropyl-β-cyclodextrin (HP-β-CD) inclusion complexes by solution-enhanced dispersion with supercritical CO2 (SEDS) and optimize process parameters, in vitro dissolution evaluation, and determination of inclusion sites.
Methods
The effects of operating pressure, operating temperature, drug concentration, and solution flow rate on the particle size and morphology of the inclusion complex were analyzed by a single factor design experiment. The SEDS-prepared inclusion complex was characterized by TG/DSC, XRD, SEM, FT-IR, 1H NMR, 2D-ROESY, and MD and measured for in vitro dissolution, solubility, and antibacterial activity.
Results
The optimum drug concentration was 40 mg/mL and pressure 16 MPa, temperature 35 °C, and solution flow rate 1 mL/min; under this condition, the mean particle size of the inclusion complex was 1.91 μm. All characterization results confirmed the formation of an amorphous inclusion complex and the sites where TFLX and HP-β-CD bind through the H-bond were located on the aromatic B ring, pyrrolidine, and naphthyridine ring protons. Furthermore, the solubility of the inclusion complex (489.87 μg/mL) was significantly higher than that of TFLX, and the dissolution rate of TFLX increased from the initial 13.99 to 61.04% in ultrapure water. In vitro study showed that the inclusion complex maintained the antibacterial effect of TFLX.
Conclusion
TFLX/HP-β-CD inclusion complex prepared by manipulating SEDS process conditions could significantly improve the dissolution and solubility of the water-insoluble TFLX.











Similar content being viewed by others
Abbreviations
- TFLX:
-
Tosufloxacin tosylate
- HP-β-CD:
-
Hydroxypropyl-β-cyclodextrin
- CDs:
-
Cyclodextrins
- DMF:
-
N,N-dimethylformamide
- EtOH:
-
Ethanol
- MeOH:
-
Methanol
- SEDS:
-
Solution-enhanced dispersion by supercritical fluids
- SEM:
-
Scanning electron microscopy
- TG/DSC:
-
Thermogravimetric/differential scanning calorimetry
- XRD:
-
X-ray diffractometry
- FT-IR:
-
Fourier transform infrared spectroscopy
- 1H NMR and 2D ROESY:
-
1H nuclear magnetic resonance and 2D rotating-frame Overhauser effect spectroscopy
- MD:
-
Molecular docking simulations
- S. aureus :
-
Staphylococcus aureus
- E. coli :
-
Escherichia coli
References
Rodriguez-Aller M, Guillarme D, Veuthey JL, Gurny R. Strategies for formulating and delivering poorly water-soluble drug. J Drug Deliv Sci Technol. 2015;30:342–51.
Sievens-Figueroa L, Bhakay A, Jerez-Rozo JI, Pandya N, Romanach RJ, Michniak-Kohn B, et al. Preparation and characterization of hydroxypropyl methyl cellulose films containing stable BCS class II drug nanoparticles for pharmaceutical applications. Int J Pharm. 2012;423:496–508.
Takeuchi N, Ohkusu M, Hoshino T, Naito S, Takaya A, Yamamoto T, et al. Emergence of quinolone-resistant strains in Streptococcus pneumoniae isolated from paediatric patients since the approval of oral fluoroquinolones in Japan. J Infect Chemother. 2017;23:218–23.
Takahata M, Nishino T. Antibacterial activities of tosufloxacin against anaerobic bacteria and the electron micrograph of its bactericidal effects. Chemotherapy. 1997;43:153–8.
Barry AL, Fuchs PC. In vitro activities of sparfloxacin, tosufloxacin, ciprofloxacin, and fleroxacin. Antimicrob Agents Chemother. 1991;35:955–60.
Norrby SR. New fluoroquinolones: towards expanded indications. Curr Opin Infect Dis. 1997;10:440–3.
Yoshida K, Kobayashi N, Saitoh H, Negishi T, Yamada T, Watanabe T, et al. Clinical efficacy of tosufloxacin on the patients with urinary tract infections. Hinyokika Kiyo. 1992;38:129–34.
Mikamo H, Yamagishi Y, Tanaka K, Watanabe K, Fujiwara M, Iwasaku K, et al. Efficacy of tosufloxacin in genital chlamydial infections. Jap J Antibiot. 2009;63:406–14.
George RC, Uttley AHC. Susceptibility of enterococci and epidemiology of enterococcal infection in the 1980s. Epidemiol Infect. 1989;103:403–13.
Chin NX, Neu HC. In-vitro activity of WIN 57273 compared to the activity of the fluoroquinolones and two β-lactam antibiotics. J Antimicrob Chemother. 1991;27:781–91.
Chu DT, Lico IM, Swanson RN, Marsh KC, Plattner JJ, Pernet AG. Synthesis and biological properties of A-71497: a prodrug of tosufloxacin. Drugs Exp Clin Res. 1990;16:435–43.
Yuan ZB, Guo P. Study of determination method of dissolution of tosufloxacin tosylate capsules. Chin J Pharm Pract. 1998;16:157–9.
Christian Leuner JD. Improving drug solubility for oral delivery using solid dispersions. Eur J Pharm Biopharm. 2000;50:47–60.
Jena SK, Singh C, Dora CP, Suresh S. Development of tamoxifen-phospholipid complex: novel approach for improving solubility and bioavailability. Int J Pharm. 2014;473:1–9.
Michael VV, Rodriguez J, Jamison JA, Borromeo PS, Turner WW. The synthesis of water soluble prodrugs analogs of echinocandin. B Bioorg Med Chem Lett. 1999;9:1863–8.
Bora PB, Bhise K. Formulation and evaluation of self micro emulsifying drug delivery system of low solubility drug for enhanced solubility and dissolution. Asian J Biomed Pharm Sci. 2013;2:7–14.
Merisko-Liversidge EM, Liversidge GG. Drug nanoparticles: formulating poorly water-soluble compounds. Toxicol Pathol. 2008;36:43–8.
Zhao MR, Wang LS, Li HW, Wang YJ, Yang H. Preparation, physicochemical characterization and in vitro dissolution studies of azithromycin-cyclodextrin inclusion complexes. J Incl Phenom Macrocycl Chem. 2016;85:137–49.
Loftsson T, Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11.
Li Y, Zhen W. Preparation, structure and performance of poly(lactic acid)/poly(lactic acid)-γ-Cyclodextrin inclusion complex-poly (glycidyl methacrylate) composites. Macromol Res. 2018;26:215–25.
Wang H, Xie X, Zhang F, Zhou Q, Tao Q, Zou Y, et al. Evaluation of cholesterol depletion as a marker of nephrotoxicity in vitro for novel beta-cyclodextrin derivatives. Food Chem Toxicol. 2011;49:1387–93.
Wang L, Yan J, Li Y, Xu K, Li S. The influence of hydroxypropyl-beta-cyclodextrin on the solubility, dissolution, cytotoxicity, and binding of riluzole with human serum albumin. J Pharm Biomed Anal. 2016;117:453–63.
Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–35.
Hong HL, Sun JF, Zhang Y, Zhu N, Han LM, Suo QL. Preparation, characterization and in vitro evaluation of tosufloxacin tosylate and hydroxypropyl-beta-cyclodextrin inclusion complex. Indian J Pharm Sci. 2019;81:249–58.
Ahn BK, Lee SG, Kim SR, Lee DH, Oh MH, Lee MW, et al. Inclusion compound formulation of hirsutenone with beta-cyclodextrin. J Pharm Investig. 2013;43:453–9.
Nishikawa S, Kondo M, Kamimura E. Ultrasonic relaxation associated with inclusion complex of drugs and β-cyclodextrin. Bull Chem Soc Jpn. 2007;80:694–8.
Celebioglu A, Uyar T. Electrospinning of polymer-free nanofibers from cyclodextrin inclusion complexes. Langmuir. 2011;27:6218–26.
Oguchi T, Okada M, Yonemochi E, Yamamoto K, Nakai Y. Freeze-drying of drug-additive binary systems III. Crystallization of α-cyclodextrin inclusion complex in freezing process. Int J Pharm. 1990;61:27–34.
Luciana WPO, Fernandes P, Sztatisz J, Szilágyi IM. Solid state studies on molecular inclusions of Lippia sidoides essential oil obtained by spray drying. J Therm Anal Calorim. 2009;95:855–63.
Lee CW, Kim SJ, Youn YS, Widjojokusumo E, Lee YH, Kim J, et al. Preparation of bitter taste masked cetirizine dihydrochloride/β-cyclodextrin inclusion complex by supercritical antisolvent (SAS) process. J Supercrit Fluids. 2010;55:348–57.
Chen AZ, Li L, Wang SB, Zhao C, Liu YG. Nanonization of methotrexate by solution-enhanced dispersion by supercritical CO2. J Supercrit Fluids. 2012;67:7–13.
Banchero M, Manna L. Investigation of the piroxicam/hydroxypropyl-β-cyclodextrin inclusion complexation by means of a supercritical solvent in the presence of auxiliary agents. J Supercrit Fluids. 2011;57:259–66.
Matos RL, Lu TJ, Prosapio V, McConville C, Leeke G,Ingram A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J CO2 Util. 2019;30:48–62.
Kaga K, Honda M, Adachi T, Honjo M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): effect of PVP and astaxanthin Z-isomer content. J Supercrit Fluids. 2018;136:44–51.
Adeoye O, Costa C, Casimiro T, Aguiar-Ricardo A, Marques HC. Preparation of ibuprofen/hydroxypropyl-γ-cyclodextrin inclusion complexes using supercritical CO2-assisted spray drying. J Supercrit Fluids. 2018;133:479–85.
Miletic T, Kyriakos K, Graovac A, Ibric S. Spray-dried voriconazole-cyclodextrin complexes: solubility, dissolution rate and chemical stability. Carbohydr Polym. 2013;98:122–31.
Michalska P, Wojnicz A, Ruiz-Nuño A, Abril S. Inclusion complex of ITH12674 with 2-hydroxypropyl-β-cyclodextrin: preparation, physical characterization and pharmacological effect. Carbohydr Polym. 2017;157:94–104.
Zhao MM, Wang HY, Yang B, Tao H. Identification of cyclodextrin inclusion complex of chlorogenic acid and its antimicrobial activity. Food Chem. 2010;120:1138–42.
Reverchon E, Marco ID. Supercritical antisolvent precipitation of cephalosporins. Powder Technol. 2006;164:139–46.
Sui X, Wei W, Yang L, Zu Y, Zhao C. Preparation, characterization and in vivo assessment of the bioavailability of glycyrrhizic acid microparticles by supercritical anti-solvent process. Int J Pharm. 2013;423:471–9.
Miao H, Chen Z, Xu W, Wang W, Song Y, Wang Z. Preparation and characterization of naringenin microparticles via a supercritical anti-solvent process. J Supercrit Fluids. 2017;131:19–25.
Hong HL, Suo QL, Li FW, Wei XH, Zhang JB. Precipitation and characterization of chelerythrine microparticles by the supercritical antisolvent process. Chem Eng Technol. 2008;31:1051–5.
Erriguible A, Fadli T, Subra-Paternault P. A complete 3D simulation of a crystallization process induced by supercritical CO2 to predict particle size. Comput Chem Eng. 2013;52:1–9.
Lengsfeld R, Delplanque JP, Barocas VH, Randolph TW. Mechanism governing microparticle morphology during precipitation by a compressed antisolvent: atomization vs nucleation and growth. J Phys Chem B. 2000;104:2725–35.
Wei Y, Zhang J, Zhou Y, Bei W, Li Y, Yuan Q, et al. Characterization of glabridin/hydroxypropyl-β-cyclodextrin inclusion complex with robust solubility and enhanced bioactivity. Carbohydr Polym. 2017;159:152–60.
Acknowledgments
The authors are very grateful to the National Natural Science Foundation of China (Grant No. 21666026) and the Natural Science Foundation of Inner Mongolia (Grant No. 2015MS0204) for their financial support in the experimental work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOC 27055 kb)
Rights and permissions
About this article
Cite this article
Sun, J., Hong, H., Zhu, N. et al. Spectroscopic Analysis and Dissolution Properties Study of Tosufloxacin Tosylate/Hydroxypropyl-β-Cyclodextrin Inclusion Complex Prepared by Solution-Enhanced Dispersion with Supercritical CO2. J Pharm Innov 15, 603–616 (2020). https://doi.org/10.1007/s12247-019-09405-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-019-09405-4