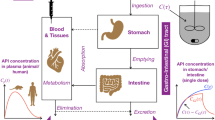
Abstract
Dissolution has become an indispensable tool to predict the in vivo performance of dosage form, especially in recent times, because of the increasing complexity of new drugs discovered. Several attempts have been made to modify dissolution, so that it mimics the in vivo behavior to maximum possible accuracy and minimizes the probability of in vivo bioequivalence failures. In this context, several advancements have been reported including drug dissolution/absorption simulating system, bionic system, dissolution/permeation model, biphasic dissolution system, Caco-2 cell monolayer in combination with compendial dissolution apparatus, artificial stomach duodenum model, dynamic gastric model, Netherlands Organization for Applied Scientific Research gastrointestinal model, and many more. The present review highlights the recent advancements in dissolution methods with a focus on in vivo predictive dissolution methods, their advantages and disadvantages, and key factors governing the results obtained. The impact of maintaining sink conditions and use of biorelevant media is also discussed briefly.













Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- ASD:
-
Artificial stomach–duodenum
- AUC:
-
Area under the curve
- BA/BE:
-
Bioavailability/bioequivalence
- BCS:
-
Biopharmaceutical classification system
- Caco:
-
Colorectal adenocarcinoma
- DDC:
-
Drug dissolution chip
- DGM:
-
Dynamic gastric model
- D/P:
-
Dissolution/permeation
- FDA:
-
Food and Drug Administration
- GIS:
-
Gastrointestinal tract simulator
- GIT:
-
Gastrointestinal tract
- iPD:
-
In vivo predictive dissolution
- IVIVC:
-
In vitro–in vivo correlation
- M-D/P:
-
Microdialysis–dissolution/permeation
- QbD:
-
Quality by design
- QCM:
-
Quartz crystal microbalance
- R&D:
-
Research and development
- SSDDs:
-
Supersaturated drug delivery systems
- TIM:
-
TNO intestinal model
- TIMagc:
-
TIM advanced gastric compartment
- USP:
-
United States Pharmacopeia
- UV:
-
Ultraviolet
References
Culen M, Dohnal J. Advances in dissolution instrumentation and their practical applications. Drug Dev Ind Pharm. 2014;40(10):1277–82. https://doi.org/10.3109/03639045.2013.841184.
USFDA. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. In: U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER), editor. 2017.
Patadia R, Vora C, Mittal K, Mashru R. Dissolution criticality in developing solid oral formulations: from inception to perception. Crit Rev Ther Drug Carrier Syst. 2013;30(6):495–534.
USFDA. Guidance for industry; immediate release solid oral dosage forms: scale-up and postapproval changes: chemistry, manufacturing, and controls, in vitro dissolution testing, and in vivo bioequivalence documentation. In: U.S. Department of Health and Human Services FaDA, Center for Drug Evaluation and Research (CDER), editor. 1995.
Grignard E, Taylor R, McAllister M, Box K, Fotaki N. Considerations for the development of in vitro dissolution tests to reduce or replace preclinical oral absorption studies. Eur J Pharm Sci. 2017;99:193–201. https://doi.org/10.1016/j.ejps.2016.12.004.
Dokoumetzidis A, Macheras P. A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm. 2006;321(1–2):1–11. https://doi.org/10.1016/j.ijpharm.2006.07.011.
Gray V, Kelly G, Xia M, Butler C, Thomas S, Mayock S. The science of USP 1 and 2 dissolution: present challenges and future relevance. Pharm Res. 2009;26(6):1289–302. https://doi.org/10.1007/s11095-008-9822-x.
Charkoftaki G, Dokoumetzidis A, Valsami G, Macheras P. Biopharmaceutical classification based on solubility and dissolution: a reappraisal of criteria for hypothesis models in the light of the experimental observations. Basic Clin Pharmacol Toxicol. 2010;106(3):168–72. https://doi.org/10.1111/j.1742-7843.2009.00506.x.
Blanquet S, Zeijdner E, Beyssac E, Meunier J-P, Denis S, Havenaar R. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm Res. 2004;21(4):585–91. https://doi.org/10.1023/B:PHAM.0000022404.70478.4b.
Kostewicz ES, Abrahamsson B, Brewster M, Brouwers J, Butler J, Carlert S, et al. In vitro models for the prediction of in vivo performance of oral dosage forms. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2014;57:342–66. https://doi.org/10.1016/j.ejps.2013.08.024.
Higashino H, Hasegawa T, Yamamoto M, Matsui R, Masaoka Y, Kataoka M, et al. In vitro-in vivo correlation of the effect of supersaturation on the intestinal absorption of BCS class 2 drugs. Mol Pharm. 2014;11(3):746–54. https://doi.org/10.1021/mp400465p.
Fong SY, Bauer-Brandl A, Brandl M. Oral bioavailability enhancement through supersaturation: an update and meta-analysis. Expert Opin Drug Deliv. 2017;14(3):403–26. https://doi.org/10.1080/17425247.2016.1218465.
Lee DH, Yeom DW, Song YS, Cho HR, Choi YS, Kang MJ, et al. Improved oral absorption of dutasteride via Soluplus®-based supersaturable self-emulsifying drug delivery system (S-SEDDS). Int J Pharm. 2015;478(1):341–7. https://doi.org/10.1016/j.ijpharm.2014.11.060.
Martinez LM, Videa M, Lopez Silva T, Castro S, Caballero A, Lara-Diaz VJ, et al. Two-phase amorphous-amorphous solid drug dispersion with enhanced stability, solubility and bioavailability resulting from ultrasonic dispersion of an immiscible system. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2017;119:243–52. https://doi.org/10.1016/j.ejpb.2017.06.021.
Laine AL, Price D, Davis J, Roberts D, Hudson R, Back K, et al. Enhanced oral delivery of celecoxib via the development of a supersaturable amorphous formulation utilising mesoporous silica and co-loaded HPMCAS. Int J Pharm. 2016;512(1):118–25. https://doi.org/10.1016/j.ijpharm.2016.08.034.
Sanphui P, Devi VK, Clara D, Malviya N, Ganguly S, Desiraju GR. Cocrystals of hydrochlorothiazide: solubility and diffusion/permeability enhancements through drug-coformer interactions. Mol Pharm. 2015;12(5):1615–22. https://doi.org/10.1021/acs.molpharmaceut.5b00020.
Van Den Abeele J, Brouwers J, Mattheus R, Tack J, Augustijns P. Gastrointestinal behavior of weakly acidic BCS class II drugs in man—case study of diclofenac potassium. J Pharm Sci. 2016;105(2):687–96. https://doi.org/10.1002/jps.24647.
Hamed R, Alnadi SH. Transfer behavior of the weakly acidic BCS class II drug valsartan from the stomach to the small intestine during fasted and fed states. AAPS PharmSciTech. 2018. https://doi.org/10.1208/s12249-018-1028-x.
Tsume Y, Mudie DM, Langguth P, Amidon GE, Amidon GL. The biopharmaceutics classification system: subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2014;57:152–63. https://doi.org/10.1016/j.ejps.2014.01.009.
Alvarez C, Nunez I, Torrado JJ, Gordon J, Potthast H, Garcia-Arieta A. Investigation on the possibility of biowaivers for ibuprofen. J Pharm Sci. 2011;100(6):2343–9. https://doi.org/10.1002/jps.22472.
Hamed R, Awadallah A, Sunoqrot S, Tarawneh O, Nazzal S, AlBaraghthi T, et al. pH-dependent solubility and dissolution behavior of carvedilol—case example of a weakly basic BCS class II drug. AAPS PharmSciTech. 2016;17(2):418–26. https://doi.org/10.1208/s12249-015-0365-2.
Tsume Y, Takeuchi S, Matsui K, Amidon GE, Amidon GL. In vitro dissolution methodology, mini-gastrointestinal simulator (mGIS), predicts better in vivo dissolution of a weak base drug, dasatinib. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2015;76:203–12. https://doi.org/10.1016/j.ejps.2015.05.013.
Takeuchi S, Tsume Y, Amidon GE, Amidon GL. Evaluation of a three compartment in vitro gastrointestinal simulator dissolution apparatus to predict in vivo dissolution. J Pharm Sci. 2014;103(11):3416–22. https://doi.org/10.1002/jps.24112.
Galia E, Nicolaides E, Horter D, Lobenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705.
Vertzoni M, Fotaki N, Kostewicz E, Stippler E, Leuner C, Nicolaides E, et al. Dissolution media simulating the intralumenal composition of the small intestine: physiological issues and practical aspects. J Pharm Pharmacol. 2004;56(4):453–62. https://doi.org/10.1211/0022357022935.
Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm. 2005;60(3):413–7. https://doi.org/10.1016/j.ejpb.2005.03.002.
Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–76. https://doi.org/10.1007/s11095-008-9569-4.
Vertzoni M, Diakidou A, Chatzilias M, Soderlind E, Abrahamsson B, Dressman JB, et al. Biorelevant media to simulate fluids in the ascending colon of humans and their usefulness in predicting intracolonic drug solubility. Pharm Res. 2010;27(10):2187–96. https://doi.org/10.1007/s11095-010-0223-6.
Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharm. 2010;7(5):1388–405. https://doi.org/10.1021/mp100149j.
Reppas C, Karatza E, Goumas C, Markopoulos C, Vertzoni M. Characterization of contents of distal ileum and cecum to which drugs/drug products are exposed during bioavailability/bioequivalence studies in healthy adults. Pharm Res. 2015;32(10):3338–49. https://doi.org/10.1007/s11095-015-1710-6.
Koziolek M, Grimm M, Garbacz G, Kuhn JP, Weitschies W. Intragastric volume changes after intake of a high-caloric, high-fat standard breakfast in healthy human subjects investigated by MRI. Mol Pharm. 2014;11(5):1632–9. https://doi.org/10.1021/mp500022u.
Schneider F, Grimm M, Koziolek M, Modess C, Dokter A, Roustom T, et al. Resolving the physiological conditions in bioavailability and bioequivalence studies: comparison of fasted and fed state. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2016;108:214–9. https://doi.org/10.1016/j.ejpb.2016.09.009.
Hens B, Brouwers J, Anneveld B, Corsetti M, Symillides M, Vertzoni M, et al. Gastrointestinal transfer: in vivo evaluation and implementation in in vitro and in silico predictive tools. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2014;63:233–42. https://doi.org/10.1016/j.ejps.2014.07.008.
Brouwers J, Augustijns P. Resolving intraluminal drug and formulation behavior: gastrointestinal concentration profiling in humans. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2014;61:2–10. https://doi.org/10.1016/j.ejps.2014.01.010.
Koziolek M, Grimm M, Becker D, Iordanov V, Zou H, Shimizu J, et al. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap(®) system. J Pharm Sci. 2015;104(9):2855–63. https://doi.org/10.1002/jps.24274.
Sjogren E, Dahlgren D, Roos C, Lennernas H. Human in vivo regional intestinal permeability: quantitation using site-specific drug absorption data. Mol Pharm. 2015;12(6):2026–39. https://doi.org/10.1021/mp500834v.
Lennernas H, Aarons L, Augustijns P, Beato S, Bolger M, Box K, et al. Oral biopharmaceutics tools—time for a new initiative—an introduction to the IMI project OrBiTo. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2014;57:292–9. https://doi.org/10.1016/j.ejps.2013.10.012.
Margolskee A, Darwich AS, Pepin X, Pathak SM, Bolger MB, Aarons L, et al. IMI—oral biopharmaceutics tools project—evaluation of bottom-up PBPK prediction success. Part 1: characterisation of the OrBiTo database of compounds. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2017;96:598–609. https://doi.org/10.1016/j.ejps.2016.09.027.
Margolskee A, Darwich AS, Pepin X, Aarons L, Galetin A, Rostami-Hodjegan A, et al. IMI—oral biopharmaceutics tools project—evaluation of bottom-up PBPK prediction success. Part 2: an introduction to the simulation exercise and overview of results. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2017;96:610–25. https://doi.org/10.1016/j.ejps.2016.10.036.
Darwich AS, Margolskee A, Pepin X, Aarons L, Galetin A, Rostami-Hodjegan A, et al. IMI—oral biopharmaceutics tools project—evaluation of bottom-up PBPK prediction success. Part 3: identifying gaps in system parameters by analysing in silico performance across different compound classes. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2017;96:626–42. https://doi.org/10.1016/j.ejps.2016.09.037.
Santos RM. Physicochemical principles of pharmacy. Am J Pharm Educ. 2006;70(5):122.
Lin Z, Zhou D, Hoag S, Qiu Y. Influence of drug properties and formulation on in vitro drug release and biowaiver regulation of oral extended release dosage forms. AAPS J. 2016;18(2):333–45. https://doi.org/10.1208/s12248-015-9861-2.
Tres F, Patient JD, Williams PM, Treacher K, Booth J, Hughes LP, et al. Monitoring the dissolution mechanisms of amorphous bicalutamide solid dispersions via real-time Raman mapping. Mol Pharm. 2015;12(5):1512–22. https://doi.org/10.1021/mp500829v.
Zhao J, Koo O, Pan D, Wu Y, Morkhade D, Rana S, et al. The impact of disintegrant type, surfactant, and API properties on the processability and performance of roller compacted formulations of acetaminophen and aspirin. AAPS J. 2017;19(5):1387–95. https://doi.org/10.1208/s12248-017-0104-6.
Panda N, Venkateshwar Reddy A, Reddy G, Panda K. Effect of different grades of HPMC and Eudragit on drug release profile of doxofylline sustained release matrix tablets and IVIVC studies. 2015.
Narang AS, Desai D, Badawy S. Impact of excipient interactions on solid dosage form stability. Pharm Res. 2012;29(10):2660–83. https://doi.org/10.1007/s11095-012-0782-9.
Kambayashi A, Yasuji T, Dressman JB. Prediction of the precipitation profiles of weak base drugs in the small intestine using a simplified transfer (“dumping”) model coupled with in silico modeling and simulation approach. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2016;103:95–103. https://doi.org/10.1016/j.ejpb.2016.03.020.
Sheng JJ, McNamara DP, Amidon GL. Toward an in vivo dissolution methodology: a comparison of phosphate and bicarbonate buffers. Mol Pharm. 2009;6(1):29–39. https://doi.org/10.1021/mp800148u.
Dressman JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15(1):11–22.
Dahan AS, Amidon GL. Gastrointestinal dissolution and absorption of class II drugs. In: Waterbeemd DHvd, Testa PDB, editors. Drug bioavailability. 2009.
Phillips DJ, Pygall SR, Cooper VB, Mann JC. Overcoming sink limitations in dissolution testing: a review of traditional methods and the potential utility of biphasic systems. J Pharm Pharmacol. 2012;64(11):1549–59. https://doi.org/10.1111/j.2042-7158.2012.01523.x.
Li ZQ, He X, Gao X, Xu YY, Wang YF, Gu H, et al. Study on dissolution and absorption of four dosage forms of isosorbide mononitrate: level A in vitro-in vivo correlation. Europ J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2011;79(2):364–71. https://doi.org/10.1016/j.ejpb.2011.04.015.
Li ZQ, Tian S, Gu H, Wu ZG, Nyagblordzro M, Feng G, et al. In vitro-in vivo predictive dissolution-permeation-absorption dynamics of highly permeable drug extended-release tablets via drug dissolution/absorption simulating system and pH alteration. AAPS PharmSciTech. 2018;19(4):1882–93. https://doi.org/10.1208/s12249-018-0996-1.
Liu W, He X, Li Z, Gao X, Ma Y, Xun M, et al. Development of a bionic system for the simultaneous prediction of the release/absorption characteristics of enteric-coated formulations. Pharm Res. 2013;30(2):596–605. https://doi.org/10.1007/s11095-012-0905-3.
Motz SA, Schaefer UF, Balbach S, Eichinger T, Lehr CM. Permeability assessment for solid oral drug formulations based on Caco-2 monolayer in combination with a flow through dissolution cell. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2007;66(2):286–95. https://doi.org/10.1016/j.ejpb.2006.10.015.
Kataoka M, Masaoka Y, Yamazaki Y, Sakane T, Sezaki H, Yamashita S. In vitro system to evaluate oral absorption of poorly water-soluble drugs: simultaneous analysis on dissolution and permeation of drugs. Pharm Res. 2003;20(10):1674–80. https://doi.org/10.1023/A:1026107906191.
Guo M, Wang K, Qiao N, Yardley V, Li M. Investigating permeation behavior of flufenamic acid cocrystals using a dissolution and permeation system. Mol Pharm. 2018;15(9):4257–72. https://doi.org/10.1021/acs.molpharmaceut.8b00670.
Gantzsch SP, Kann B, Ofer-Glaessgen M, Loos P, Berchtold H, Balbach S, et al. Characterization and evaluation of a modified PVPA barrier in comparison to Caco-2 cell monolayers for combined dissolution and permeation testing. J Control Release: Off J Control Release Soc. 2014;175:79–86. https://doi.org/10.1016/j.jconrel.2013.12.009.
Fong SYK, Poulsen J, Brandl M, Bauer-Brandl A. A novel microdialysis-dissolution/permeation system for testing oral dosage forms: a proof-of-concept study. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2017;96:154–63. https://doi.org/10.1016/j.ejps.2016.09.018.
Buch P, Langguth P, Kataoka M, Yamashita S. IVIVC in oral absorption for fenofibrate immediate release tablets using a dissolution/permeation system. J Pharm Sci. 2009;98(6):2001–9. https://doi.org/10.1002/jps.21576.
Miyaji Y, Fujii Y, Takeyama S, Kawai Y, Kataoka M, Takahashi M, et al. Advantage of the dissolution/permeation system for estimating oral absorption of drug candidates in the drug discovery stage. Mol Pharm. 2016;13(5):1564–74. https://doi.org/10.1021/acs.molpharmaceut.6b00044.
Mizoguchi M, Kataoka M, Yokoyama K, Aihara R, Wada K, Yamashita S. Application of an in vitro dissolution/permeation system to early screening of oral formulations of poorly soluble, weakly basic drugs containing an acidic pH-modifier. J Pharm Sci. 2018;107(9):2404–10. https://doi.org/10.1016/j.xphs.2018.05.009.
Takano R, Kataoka M, Yamashita S. Integrating drug permeability with dissolution profile to develop IVIVC. Biopharm Drug Dispos. 2012;33(7):354–65. https://doi.org/10.1002/bdd.1792.
Kataoka M, Masaoka Y, Sakuma S, Yamashita S. Effect of food intake on the oral absorption of poorly water-soluble drugs: in vitro assessment of drug dissolution and permeation assay system. J Pharm Sci. 2006;95(9):2051–61. https://doi.org/10.1002/jps.20691.
Charman WN, Rogge MC, Boddy AW, Berger BM. Effect of food and a monoglyceride emulsion formulation on danazol bioavailability. J Clin Pharmacol. 1993;33(4):381–6.
Kataoka M, Yano K, Hamatsu Y, Masaoka Y, Sakuma S, Yamashita S. Assessment of absorption potential of poorly water-soluble drugs by using the dissolution/permeation system. Eur J Pharm Biopharm. 2013;85(3, Part B):1317–24. https://doi.org/10.1016/j.ejpb.2013.06.018.
Kataoka M, Sugano K, da Costa Mathews C, Wong JW, Jones KL, Masaoka Y, et al. Application of dissolution/permeation system for evaluation of formulation effect on oral absorption of poorly water-soluble drugs in drug development. Pharm Res. 2012;29(6):1485–94. https://doi.org/10.1007/s11095-011-0623-2.
Kataoka M, Fukahori M, Ikemura A, Kubota A, Higashino H, Sakuma S, et al. Effects of gastric pH on oral drug absorption: in vitro assessment using a dissolution/permeation system reflecting the gastric dissolution process. Eur J Pharm Biopharm: Off JArbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2016;101:103–11. https://doi.org/10.1016/j.ejpb.2016.02.002.
Koplin S, Kumpugdee-Vollrath M, Bauer-Brandl A, Brandl M. Surfactants enhance recovery of poorly soluble drugs during microdialysis sampling: implications for in vitro dissolution-/permeation-studies. J Pharm Biomed Anal. 2017;145:586–92. https://doi.org/10.1016/j.jpba.2017.07.022.
Levy G, Leonards JR, Procknal JA. Development of in vitro dissolution tests which correlate quantitatively with dissolution rate-limited drug absorption in man. J Pharm Sci. 1965;54(12):1719–22. https://doi.org/10.1002/jps.2600541204.
Heigoldt U, Sommer F, Daniels R, Wagner K-G. Predicting in vivo absorption behavior of oral modified release dosage forms containing pH-dependent poorly soluble drugs using a novel pH-adjusted biphasic in vitro dissolution test. Eur J Pharm Biopharm. 2010;76(1):105–11. https://doi.org/10.1016/j.ejpb.2010.05.006.
Pestieau A, Evrard B. In vitro biphasic dissolution tests and their suitability for establishing in vitro-in vivo correlations: a historical review. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2017;102:203–19. https://doi.org/10.1016/j.ejps.2017.03.019.
Chaudhary RS, SS Gangwal , Gupta VK , Shah Y, Jindal K, Khanna S. Dissolution system for nifedipine sustained release formulations. 1994.
Pillay V, Fassihi R. Evaluation and comparison of dissolution data derived from different modified release dosage forms: an alternative method. J Control Release: Off J Control Release Soc. 1998;55(1):45–55.
Gabriels M, Plaizier-Vercammen J. Design of a dissolution system for the evaluation of the release rate characteristics of artemether and dihydroartemisinin from tablets. Int J Pharm. 2004;274(1–2):245–60. https://doi.org/10.1016/j.ijpharm.2004.01.022.
Kinget R, De Greef H. In vitro assessment of drug release from semi-solid lipid matrices. Eur J Pharm Sci. 1995;3(2):105–11. https://doi.org/10.1016/0928-0987(94)00081-A.
Ngo TH, Quintens I, Roets E, Declerck PJ, Hoogmartens J. Bioavailability of different artemisinin tablet formulations in rabbit plasma—correlation with results obtained by an in vitro dissolution method. J Pharm Biomed Anal. 1997;16(2):185–9.
Stead JA, Freeman M, John EG, Ward GT, Whiting B. Ibuprofen tablets: dissolution and bioavailability studies. Int J Pharm. 1983;14(1):59–72. https://doi.org/10.1016/0378-5173(83)90114-X.
Grundy JS, Anderson KE, Rogers JA, Foster RT. Studies on dissolution testing of the nifedipine gastrointestinal therapeutic system. II. Improved in vitro-in vivo correlation using a two-phase dissolution test. J Control Release. 1997;48(1):9–17. https://doi.org/10.1016/S0168-3659(97)01638-6.
Vangani S, li X, Zhou P, Barrio M-A, Chiu R, Cauchon N et al. Dissolution of poorly water-soluble drugs in biphasic media using USP 4 and fiber optic system. 2009.
Al Durdunji A, AlKhatib HS, Al-Ghazawi M. Development of a biphasic dissolution test for deferasirox dispersible tablets and its application in establishing an in vitro-in vivo correlation. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2016;102:9–18. https://doi.org/10.1016/j.ejpb.2016.02.006.
Shi Y, Erickson B, Jayasankar A, Lu L, Marsh K, Menon R, et al. Assessing supersaturation and its impact on in vivo bioavailability of a low-solubility compound ABT-072 with a dual pH, two-phase dissolution method. J Pharm Sci. 2016;105(9):2886–95. https://doi.org/10.1016/j.xphs.2016.04.036.
Xu H, Vela S, Shi Y, Marroum P, Gao P. In vitro characterization of ritonavir drug products and correlation to human in vivo performance. Mol Pharm. 2017;14(11):3801–14. https://doi.org/10.1021/acs.molpharmaceut.7b00552.
Xu H, Krakow S, Shi Y, Rosenberg J, Gao P. In vitro characterization of ritonavir formulations and correlation to in vivo performance in dogs. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2018;115:286–95. https://doi.org/10.1016/j.ejps.2018.01.026.
Thiry J, Broze G, Pestieau A, Tatton AS, Baumans F, Damblon C, et al. Investigation of a suitable in vitro dissolution test for itraconazole-based solid dispersions. Eur J Pharm Sci. 2016;85:94–105. https://doi.org/10.1016/j.ejps.2016.02.002.
Locher K, Borghardt JM, Frank KJ, Kloft C, Wagner KG. Evolution of a mini-scale biphasic dissolution model: impact of model parameters on partitioning of dissolved API and modelling of in vivo-relevant kinetics. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2016;105:166–75. https://doi.org/10.1016/j.ejpb.2016.06.008.
Pestieau A, Krier F, Brouwers A, Streel B, Evrard B. Selection of a discriminant and biorelevant in vitro dissolution test for the development of fenofibrate self-emulsifying lipid-based formulations. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2016;92:212–9. https://doi.org/10.1016/j.ejps.2016.04.038.
Shi Y, Gao P, Gong Y, Ping H. Application of a biphasic test for characterization of in vitro drug release of immediate release formulations of celecoxib and its relevance to in vivo absorption. Mol Pharm. 2010;7(5):1458–65. https://doi.org/10.1021/mp100114a.
Frank KJ, Locher K, Zecevic DE, Fleth J, Wagner KG. In vivo predictive mini-scale dissolution for weak bases: advantages of pH-shift in combination with an absorptive compartment. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2014;61:32–9. https://doi.org/10.1016/j.ejps.2013.12.015.
Pestieau A, Lebrun S, Cahay B, Brouwers A, Streel B, Cardot JM, et al. Evaluation of different in vitro dissolution tests based on level A in vitro-in vivo correlations for fenofibrate self-emulsifying lipid-based formulations. Eur J Pharm Biopharm: Off JArbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2017;112:18–29. https://doi.org/10.1016/j.ejpb.2016.10.030.
Pestieau A, Krier F, Lebrun P, Brouwers A, Streel B, Evrard B. Optimization of a PGSS (particles from gas saturated solutions) process for a fenofibrate lipid-based solid dispersion formulation. Int J Pharm. 2015;485(1–2):295–305. https://doi.org/10.1016/j.ijpharm.2015.03.027.
Phillips DJ, Pygall S, Cooper BC. Mann J. Toward biorelevant dissolution: application of a biphasic dissolution model as a discriminating tool for HPMC matrices containing a model BCS class II drug. 2012.
Xu H, Shi Y, Vela S, Marroum P, Gao P. Developing quantitative in vitro-in vivo correlation for fenofibrate immediate-release formulations with the biphasic dissolution-partition test method. J Pharm Sci. 2018;107(1):476–87. https://doi.org/10.1016/j.xphs.2017.06.018.
Law D, Schmitt EA, Marsh KC, Everitt EA, Wang W, Fort JJ, et al. Ritonavir-PEG 8000 amorphous solid dispersions: in vitro and in vivo evaluations. J Pharm Sci. 2004;93(3):563–70. https://doi.org/10.1002/jps.10566.
Carino SR, Sperry DC, Hawley M. Relative bioavailability estimation of carbamazepine crystal forms using an artificial stomach-duodenum model. J Pharm Sci. 2006;95(1):116–25. https://doi.org/10.1002/jps.20495.
Carino SR, Sperry DC, Hawley M. Relative bioavailability of three different solid forms of PNU-141659 as determined with the artificial stomach-duodenum model. J Pharm Sci. 2010;99(9):3923–30. https://doi.org/10.1002/jps.22236.
Polster CS, Atassi F, Wu SJ, Sperry DC. Use of artificial stomach-duodenum model for investigation of dosing fluid effect on clinical trial variability. Mol Pharm. 2010;7(5):1533–8. https://doi.org/10.1021/mp100116g.
Polster CS, Wu SJ, Gueorguieva I, Sperry DC. Mechanism for enhanced absorption of a solid dispersion formulation of LY2300559 using the artificial stomach duodenum model. Mol Pharm. 2015;12(4):1131–40. https://doi.org/10.1021/mp5006036.
Bhattachar SN, Perkins EJ, Tan JS, Burns LJ. Effect of gastric pH on the pharmacokinetics of a BCS class II compound in dogs: utilization of an artificial stomach and duodenum dissolution model and GastroPlus, simulations to predict absorption. J Pharm Sci. 2011;100(11):4756–65. https://doi.org/10.1002/jps.22669.
Tsume Y, Amidon GL, Takeuchi S. Dissolution effect of gastric and intestinal pH for a BCS class II drug, pioglitazone: new in vitro dissolution system to predict in vivo dissolution. J Bioequivalence Bioavailability. 2013;5(6):224–7. https://doi.org/10.4172/jbb.1000162.
Matsui K, Tsume Y, Amidon GE, Amidon GL. In vitro dissolution of fluconazole and dipyridamole in gastrointestinal simulator (GIS), predicting in vivo dissolution and drug-drug interaction caused by acid-reducing agents. Mol Pharm. 2015;12(7):2418–28. https://doi.org/10.1021/acs.molpharmaceut.5b00135.
Matsui K, Tsume Y, Amidon GE, Amidon GL. The evaluation of in vitro drug dissolution of commercially available oral dosage forms for itraconazole in gastrointestinal simulator with biorelevant media. J Pharm Sci. 2016;105(9):2804–14. https://doi.org/10.1016/j.xphs.2016.02.020.
Christopher LJ, Cui D, Wu C, Luo R, Manning JA, Bonacorsi SJ, et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos:Biol Fate Chem. 2008;36(7):1357–64. https://doi.org/10.1124/dmd.107.018267.
Tsume Y, Igawa N, Drelich AJ, Amidon GE, Amidon GL. The combination of GIS and biphasic to better predict in vivo dissolution of BCS class IIb drugs, ketoconazole and raloxifene. J Pharm Sci. 2018;107(1):307–16. https://doi.org/10.1016/j.xphs.2017.09.002.
Matsui K, Tsume Y, Takeuchi S, Searls A, Amidon GL. Utilization of gastrointestinal simulator, an in vivo predictive dissolution methodology, coupled with computational approach to forecast oral absorption of dipyridamole. 2017;14(4):1181–9. https://doi.org/10.1021/acs.molpharmaceut.6b01063.
Psachoulias D, Vertzoni M, Goumas K, Kalioras V, Beato S, Butler J, et al. Precipitation in and supersaturation of contents of the upper small intestine after administration of two weak bases to fasted adults. Pharm Res. 2011;28(12):3145–58. https://doi.org/10.1007/s11095-011-0506-6.
Russell TL, Berardi RR, Barnett JL, O’Sullivan TL, Wagner JG, Dressman JB. pH-related changes in the absorption of dipyridamole in the elderly. Pharm Res. 1994;11(1):136–43.
Thuenemann EC, Mandalari G, Rich GT, Faulks RM. Dynamic gastric model (DGM). In: Verhoeckx K, CotterIván P, López-Expósito I, Kleiveland C, Lea T, Mackie A, et al., editors. The impact of food bioactives on health; 2018.
Marciani L, Gowland PA, Fillery-Travis A, Manoj P, Wright J, Smith A, et al. Assessment of antral grinding of a model solid meal with echo-planar imaging. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G844–9. https://doi.org/10.1152/ajpgi.2001.280.5.G844.
Vardakou M, Mercuri A, Barker SA, Craig DQM, Faulks RM, Wickham MSJ. Achieving antral grinding forces in biorelevant in vitro models: comparing the USP dissolution apparatus II and the dynamic gastric model with human in vivo data. AAPS PharmSciTech. 2011;12(2):620–6. https://doi.org/10.1208/s12249-011-9616-z.
Vardakou M, Mercuri A, Naylor TA, Rizzo D, Butler JM, Connolly PC, et al. Predicting the human in vivo performance of different oral capsule shell types using a novel in vitro dynamic gastric model. Int J Pharm. 2011;419(1–2):192–9. https://doi.org/10.1016/j.ijpharm.2011.07.046.
Mann JC, Pygall SR. A formulation case study comparing the dynamic gastric model with conventional dissolution methods. Dissolution Technologies. 2012;19(4):14–9.
Chessa S, Huatan H, Levina M, Mehta RY, Ferrizzi D, Rajabi-Siahboomi AR. Application of the dynamic gastric model to evaluate the effect of food on the drug release characteristics of a hydrophilic matrix formulation. Int J Pharm. 2014;466(1–2):359–67. https://doi.org/10.1016/j.ijpharm.2014.03.031.
Mason LM, Chessa S, Huatan H, Storey DE, Gupta P, Burley J, et al. Use of the dynamic gastric model as a tool for investigating fed and fasted sensitivities of low polymer content hydrophilic matrix formulations. Int J Pharm. 2016;510(1):210–20. https://doi.org/10.1016/j.ijpharm.2016.06.034.
Minekus M, Marteau P, Havenaar R, Huis in 't Veld J. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. ATLA Altern Lab Anim 1995.
Dickinson PA, Abu Rmaileh R, Ashworth L, Barker RA, Burke WM, Patterson CM, et al. An investigation into the utility of a multi-compartmental, dynamic, system of the upper gastrointestinal tract to support formulation development and establish bioequivalence of poorly soluble drugs. AAPS J. 2012;14(2):196–205. https://doi.org/10.1208/s12248-012-9333-x.
Cardot J-M, Beyssac E, Alric M. In vitro–in vivo correlation: importance of dissolution in IVIVC. Dissolution Technologies. 2007;14(1):15–9.
Nimalaratne C, Savard P, Gauthier SF, Schieber A, Wu J. Bioaccessibility and digestive stability of carotenoids in cooked eggs studied using a dynamic in vitro gastrointestinal model. J Agric Food Chem. 2015;63(11):2956–62. https://doi.org/10.1021/jf505615w.
Van Loo-Bouwman CA, Naber THJ, Minekus M, van Breemen RB, Hulshof PJM, Schaafsma G. Food matrix effects on bioaccessibility of β-carotene can be measured in an in vitro gastrointestinal model. J Agric Food Chem. 2014;62(4):950–5. https://doi.org/10.1021/jf403312v.
Havenaar R, de Jong A, Koenen ME, van Bilsen J, Janssen AM, Labij E, et al. Digestibility of transglutaminase cross-linked caseinate versus native caseinate in an in vitro multicompartmental model simulating young child and adult gastrointestinal conditions. J Agric Food Chem. 2013;61(31):7636–44. https://doi.org/10.1021/jf402824u.
Tenjarla S, Romasanta V, Zeijdner E, Villa R, Moro L. Release of 5-aminosalicylate from an MMX mesalamine tablet during transit through a simulated gastrointestinal tract system. Adv Ther. 2007;24(4):826–40.
Souliman S, Beyssac E, Cardot JM, Denis S, Alric M. Investigation of the biopharmaceutical behavior of theophylline hydrophilic matrix tablets using USP methods and an artificial digestive system. Drug Dev Ind Pharm. 2007;33(4):475–83. https://doi.org/10.1080/03639040601128654.
Brouwers J, Anneveld B, Goudappel GJ, Duchateau G, Annaert P, Augustijns P, et al. Food-dependent disintegration of immediate release fosamprenavir tablets: in vitro evaluation using magnetic resonance imaging and a dynamic gastrointestinal system. Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2011;77(2):313–9. https://doi.org/10.1016/j.ejpb.2010.10.009.
Reis PM, Raab TW, Chuat JY, Leser ME, Miller R, Watzke HJ, et al. Influence of surfactants on lipase fat digestion in a model gastro-intestinal system. Food Biophysics. 2008;3(4):370–81. https://doi.org/10.1007/s11483-008-9091-6.
Van Den Abeele J, Schilderink R, Schneider F, Mols R, Minekus M, Weitschies W, et al. Gastrointestinal and systemic disposition of diclofenac under fasted and fed state conditions supporting the evaluation of in vitro predictive tools. 2017;14(12):4220–32. https://doi.org/10.1021/acs.molpharmaceut.7b00253.
Gulati S, Bonoan JA, Schesser K V, Arucan JF, Li X. Microfluidic measurements of drug dissolution using a quartz crystal microbalance. ASME 2016 14th Int. Conf. Nanochannels, Microchannels, Minichannels collocated with ASME 2016 Heat Transf. Summer Conf. ASME 2016 Fluids Eng. Div. Summer Meet. Am Soc Mech Eng. 2016. p. V001T10A001-V001T10A001.
Windbergs M, Weitz DA. Drug dissolution chip (DDC): a microfluidic approach for drug release. Small (Weinheim an der Bergstrasse, Germany). 2011;7(21):3011–5. https://doi.org/10.1002/smll.201100520.
Chi CW, Ahmed AR, Dereli-Korkut Z, Wang S. Microfluidic cell chips for high-throughput drug screening. Bioanalysis. 2016;8(9):921–37. https://doi.org/10.4155/bio-2016-0028.
Batchelor HK, Fotaki N, Klein S. Paediatric oral biopharmaceutics: key considerations and current challenges. Adv Drug Deliv Rev. 2014;73:102–26. https://doi.org/10.1016/j.addr.2013.10.006.
Karkossa F, Krueger A, Urbaniak J, Klein S. Simulating different dosing scenarios for a child-appropriate valproate ER formulation in a new pediatric two-stage dissolution model. AAPS PharmSciTech. 2017;18(2):309–16. https://doi.org/10.1208/s12249-016-0671-3.
Guidance for industry: extended release oral dosage forms: development, evaluation, and application of in vitro/in vivo correlations, (1997).
Nguyen MA, Flanagan T, Brewster M, Kesisoglou F, Beato S, Biewenga J, et al. A survey on IVIVC/IVIVR development in the pharmaceutical industry—past experience and current perspectives. Eur J Pharm Sci: Off J Eur Fed Pharm Sci. 2017;102:1–13. https://doi.org/10.1016/j.ejps.2017.02.029.
Dickinson PA, Lee WW, Stott PW, Townsend AI, Smart JP, Ghahramani P, et al. Clinical relevance of dissolution testing in quality by design. AAPS J. 2008;10(2):380–90. https://doi.org/10.1208/s12248-008-9034-7.
Polli JE, Cook JA, Davit BM, Dickinson PA, Argenti D, Barbour N, et al. Summary workshop report: facilitating oral product development and reducing regulatory burden through novel approaches to assess bioavailability/bioequivalence. AAPS J. 2012;14(3):627–38. https://doi.org/10.1208/s12248-012-9376-z.
Acknowledgments
The authors want to thank the Ministry of Chemicals and Fertilizers for providing the funding and the Department of Science and Technology for providing the funding to authors in the form of INSPIRE faculty award (Grant number LSBM-13; SERB EMR/2016/007966).
Funding
This work was supported by the National Institute of Pharmaceutical Education and Research (NIPER), Ahmedabad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2377 kb)
Rights and permissions
About this article
Cite this article
Shrivas, M., Khunt, D., Shrivas, M. et al. Advances in In Vivo Predictive Dissolution Testing of Solid Oral Formulations: How Closer to In Vivo Performance?. J Pharm Innov 15, 296–317 (2020). https://doi.org/10.1007/s12247-019-09392-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-019-09392-6