Abstract
In the present study, the evolution of the physicochemical and microbiological characteristics of lactic acid bacteria (LAB) in traditional Kırklareli white brined cheese collected from 14 different cheese manufacturing facilities were investigated on different days of the 90-day ripening period. The obtained LAB within the species Lactococcus (Lc.) lactis, Latilactobacillus (Lt.) curvatus, Lactobacillus (Lb.) casei and Lb. plantarum, Enterococcus (E.) durans, E. faecium, E. faecalis, Streptococcus macedonicus, and Weissella paramesenteroides were characterized in terms of their influence on technological properties and their potential as starter cultures for traditional white brined cheese production. The results of the microbiological and physicochemical investigations showed that a few selected isolates of Lc. lactis, Lb. casei, and Lb. plantarum had certain functions as starter germs. Moderate acidification capacity, antibacterial activity and proteolytic activity, which are characteristic of their use as starter lactic acid bacteria, were found. Importantly, antibiotic resistance among selected Lc. lactis, Lb. casei, and Lb. plantarum isolates was extremely low, whereas some of these isolates demonstrated antibacterial activity against major foodborne pathogenic bacteria. Based on the results obtained in this study, selected Lc. and Lb. isolates can also be considered as starter culture in traditional cheese production.
Similar content being viewed by others
Introduction
Manufacturing of local dairy products is a tradition that has been preserved for centuries. The products have been characterized by great diversity, and some of them have been known since ancient times. All cheeses from a certain geographic area represent a potential national treasure and cultural heritage (Terzić-Vidojević et al. 2020).
Exemplary for this are white brined cheeses, which are produced especially in the Balkans and in Mediterranean countries such as Turkey, Egypt, and Greece. This type of cheese is matured in brine (10–18% NaCl) for a long period. The level of salt in the brine is crucial for selective properties thereof for microorganisms (Albayrak and Duran 2021). In principle, flavor, texture, and preservative properties of many fermented foods such as cheese are determined by the use of different species of the five major LAB genera: Lactobacillus spp., Lactococcus spp., Leuconostoc spp., Enterococcus spp., and Streptococcus spp. LAB are a heterogeneous group of gram-positive bacteria with a strictly fermentative metabolism, of which lactic acid is the most important metabolite (Temmerman et al. 2004). According to their specific roles, LAB involved in fermentation processes can be divided into two groups: starter lactic acid bacteria (sLAB) and non-starter LAB (nsLAB). sLAB may be added as starters and adjunct cultures. A starter is a culture of living microorganisms which are used to begin fermentation, producing specific changes in the chemical composition and sensory properties of the food product. On the other hand, nsLAB usually originate from the production and processing environments as spontaneous microbiota (Grujović et al. 2021).
In the traditional production method, in contrast to the basic principle using the five major LAB, a high proportion of various nsLAB can be observed during the ripening period, depending on the type of cheese. The presence of various microbial groups could influence the lipolysis and proteolysis process and therefore ultimately the ripening characteristics of the specific cheeses (Öner et al. 2006).
In traditionally produced cheeses, such as matured or fermented white cheese, a wide variety of LAB and various other specific bacteria can be found, which have a wide variety of functions or also act as indicator bacteria.
Therefore, the aim of this study was (i) to investigate the physicochemical and microbiological properties of traditional Kırklareli white brined cheese from local dairies during the 3-month ripening period, (ii) to characterize the obtained LAB and their influence on the technological properties, and (iii) to determine their potential as starter cultures for traditional cheese production.
Material and methods
Collection of cheese samples
In this study, Kırklareli white brined cheese samples (n = 56) were taken from 14 different cheese manufacturing facilities with a daily production volume varying from 10 to 70 tons of cheese in Kırklareli Province, Turkey. The samples were received prior to the packaging process and delivered to the laboratory at the Department of Food Engineering of Kırklareli University under suitable conditions. From each factory, four mold cheese samples (weighing approximately 650 g) were taken, one of which was tested for analysis directly on the first day. When the pH of the samples reached 5.0, the samples were transferred to 1 L containers and stored in their own brine at 4 °C during the ripening period. The ripening of the samples took place over a 3-month period. Physicochemical and microbiological analyses were carried out at days 1, 15, 30, and 90 of the ripening period. The overall study design is presented in the study design section.
Physicochemical and microbiological analysis
The acidity was determined by titration, and a salt analysis was carried out on the cheese samples as described previously (Dertli et al. 2012). For the microbiological analysis, a total of 56 white cheese samples were tested for the presence of Enterobacteriaceae, Escherichia (E.) coli, Staphylococcus (S.) aureus, molds, and yeasts. For this purpose, 10 g of each cheese sample was serially diluted in 90 mL of sterile saline peptone water (0.9% NaCl, 0.1% peptone (Oxoid Ltd., Basingstoke, UK), pH 7.0) and was homogenized using a Stomacher Lab-Blender 400 (Seward Medical Ltd., London, UK). Serial dilutions were used to perform the plating procedure. The appropriate agars and incubation periods were applied as follows: Enterobacteriaceae were determined using Violet-Red-Bile-Dextrose (VRBD) Agar (Merck KGaA, Darmstadt, Germany) at 37 °C and incubated aerobically for 24–48 h (ISO 2005); total count of molds and yeasts was determined by DRBC Agar (Merck) at 25 °C and incubated aerobically for 5 days (Tournas et al. 2001); Baird Parker (BP) Agar (Oxoid) with egg-yolk tellurite addition was used to determine S. aureus and was incubated aerobically for 30–48 h at 35–37 °C (Tallent et al. 2001). The number of E. coli was determined using Tryptone Bile X-Glucuronide (TBX) Agar medium (Oxoid), and the respective agar plates were incubated for 4 h at 30 °C, followed by 18 h at 44 °C. Then, the bluish-green colonies formed after aerobic incubation were evaluated as E. coli (Feng et al. 2002).
Isolation and identification of LAB and nsLAB
Isolation from the cheese samples
Appropriate dilution series of white cheese samples were plated out on M17 Agar (Merck) and De Man, Rogosa, and Sharpe (MRS) Agar (Merck) for enumeration of total viable LAB. The corresponding plates were incubated aerobically at 30 °C for 48 h for the growth of LAB. Different colonies were selected from the agar plates and subjected to gram-staining, cell morphology, and catalase reaction tests as described previously (Dertli et al. 2016).
Identification by 16S rRNA gene sequencing
After selecting the LAB isolates according to phenotypic characteristics, 31 typical isolates for each phenotype were identified as the corresponding bacterial species by sequence analysis of the 16S rRNA gene. The genomic DNA of the isolates was extracted using the Qiagen Bacterial DNA extraction kit (Vivantis Technologies Sdn Bhd, Selangor Darul Ehsan, Malaysia) in accordance with the manufacturer’s recommendations. A total of 1 μL of each genomic DNA from the isolates was used as a template for preparing each PCR reaction. Other components of the PCR approach, such as master mix and probes, had been previously prepared, and PCR analysis and sequencing were performed as described previously (Dertli et al. 2016). The obtained gene sequences were submitted to the National Center for Biotechnology Information (NCBI) BLAST database, aligned, and identified with a similarity criterion of 97–100%. Using Molecular Evolutionary Genetic Analysis (MEGA X) software, the 16S rDNA sequences of the cheese isolates were arranged to perform the phylogenetic analysis (Tamura et al. 2011). For this purpose, the neighbor-joining (NJ) method with 1000 bootstrap replicates was used and the phylogenetic tree was constructed (Saitou and Nei 1987). The partial 16S rDNA sequences of the 21 isolates identified in this study were deposited in the NCBI GenBank under accession numbers MT345607 to MT345627.
Determination of technological properties of isolates
Proteolytic activity
The levels of proteolytic activity of the isolates were determined by spectrophotometric measurement of tyrosine formation. This method has been described previously (Citti et al. 1963) and was applied in this study. The spectrophotometric measurements were performed at a wavelength of 650 nm (Shimadzu UV-120–02, Kyoto, Japan). The values obtained were compared with the results of the tyrosine standard and expressed as the tyrosine equivalent (µg/mL).
Hydrogen sulfide production
To determine the ability of the found isolates to produce hydrogen sulfide, active cultures were cultivated in Triple Sugar Iron (TSI) Agar (Oxoid) medium and incubated for 2 weeks at 30 °C. At the end of the aerobic incubation period, a blackening of the color of the medium was observed, demonstrating the production of hydrogen sulfide (Lee and Simard 1984).
Lactic acid-producing abilities
The isolates should also be tested for their ability to produce lactic acid. For this purpose, 1% inoculations were performed in skimmed milk medium (Biolife Italiana S.r.l., Milan, Italy) with 18-h active cultures. This was then incubated for 6 and 24 h, respectively, at 30 °C under aerobic conditions. To determine lactic acid development, pH values were determined at the end of the incubation period. The difference between the initial pH value of skimmed milk medium and the pH value after incubation (ΔpH) was considered in the evaluation (Sarantinopoulos et al. 2001).
Antibacterial activities
The cheese isolates were grown in MRS broth at 37 °C for 24 h under aerobic conditions, and the culture supernatants were obtained by filtration. The inhibitory effect of H2O2 was eliminated by adjusting the pH of the supernatants to 6.5 and adding catalase reagent (5 mg/mL) (Sigma, Missouri, USA). Nutrient agar media (Oxoid) containing 18-h grown pathogenic cultures of Bacillus cereus FMC 19, Escherichia coli ATCC 25922, Listeria monocytogenes RSKK 472 (serovar 1/2b), Salmonella typhimurium NRRLE 4463, and S. aureus ATCC 28213 were poured into petri dishes. Then, wells of 6 mm diameter were formed on the solidified medium. A supernatant of the isolate was placed in each well to be tested for antibacterial activity. The diameters of the inhibition zones formed were recorded after a 24-h incubation period (Dertli et al. 2016).
Antibiotic sensitivity
Antibiotic susceptibility testing of the isolates against ampicillin (AMP, 10 µg), gentamycin (GEN, 120 µg), clindamycin (CLI, 2 µg), chloramphenicol (CHL, 30 µg), vancomycin (VAN, 30 µg), kanamycin (KAN, 30 µg), streptomycin (STR, 10 µg), erythromycin (ERY, 10 µg), and tetracycline hydrochloride (TET, 30 µg) (Bioanalyse, Ankara, Turkey) was performed by disk diffusion assay on Mueller–Hinton agar (Bioanalyse) according to EFSA (2018).
Result and discussion
Physicochemical analysis of cheese samples
The salt and acidity values analyzed during the ripening period in traditional white cheese samples are visualized in Fig. 1. The percentage salt levels of the cheese samples were determined to be 2.88 ± 0.72%, 4.16 ± 0.84%, 5.13 ± 1.46%, and 5.51 ± 1.22% at days 1, 15, 30, and 90 of the ripening period, respectively. Similar to the salt levels of the cheese samples, there was an increase in the acidity of samples during the ripening period. Similar to the findings of this study, Uğur and Öner (2018) reported the average salt content of white cheese samples to be 4.29% and 5.59% at days 1 and 90 of the ripening period, respectively. Total titratable acidity (g lactic acid per 100 g cheese) of the samples was found to be 0.38 ± 0.18%, 0.43 ± 0.09%, 0.34 ± 0.14%, and 0.66 ± 0.14% at days 1, 15, 30, and 90 of the ripening period, respectively. However, the findings of the present study were similar to the previous findings reported for the white cheese samples collected from different regions. Hayaloglu et al. (2002) reported the titratable acidity of pickled white cheese samples to be between 0.37 and 3.80%. Çakmakçı and Kurt (1993) determined the titration acidity of fresh white cheese samples to be 0.37% and that of ripened ones to be 0.76% in their study. While the titration acidity of the cheese samples was lower on the first day, the acidity increased by the end of the ripening period. Fluctuations in titratable acidity in the later stages of ripening were caused by the formation of alkaline substances in the medium due to proteolysis during ripening and the change in dry matter (Öner and Sarıdağ 2019). It is thought that the lipolysis process, that is, the resulting fatty acid composition, also has an effect on the increase in acidity that occurs after the 90th day. Dağdemir et al. (2003) and Hayaloglu et al. (2005) also obtained similar phenomenon to our study.
Microbiological characteristics of cheese samples
The total contamination level given in CFU per gram cheese of E. coli, Enterobacteriaceae, Staphylococcus spp., S. aureus, and mold-yeast was determined four times during the 90-day ripening period. All results are displayed in Table 1. At the beginning of the ripening period, a total of 6 of 14 cheese samples demonstrated E. coli numbers of 2–4.6 log CFU/g, while only 1 cheese sample was positive at the end of the ripening period (day 90). The initial high level of E. coli in cheese samples at the beginning of ripening might be a result of fecal contamination and an insufficient heating process (Beuchat and Ryu 1997). According to the Turkish Food Codex (Codex 2011), the maximum E. coli numbers in white cheese should be 102 CFU/g. One cheese sample in this study was therefore unsuitable in terms of E. coli numbers. The cheese samples were also tested for the presence of Enterobacteriaceae, and counts of Enterobacteriaceae were observed between 3.5 and 6.4 log CFU/g at the first day of the ripening period (day 1). At the end of the ripening period, the lowest and highest Enterobacteriaceae numbers were 1.2 and 5.1 log CFU/g, respectively. As an indicator of the Enterobacteriaceae microbial group, high numbers might reveal poor hygiene and sanitation conditions as well as fecal contamination (Yücel and Ulusoy 2006).
The testing of Staphylococcus spp. and S. aureus numbers during the first day of the ripening period showed counts between 4.2 and 7.2 log CFU/g, and an approximate decrease of 1.5 log level CFU/g was observed in staphylococcus numbers at day 90. For S. aureus, eight samples tested negative, and in six cheese samples, S. aureus was observed with a contamination level of 3.3–5.1 log CFU/g. At the end of the ripening period, in three cheese samples, S. aureus was still observed (2.0–3.4 log CFU/g). The cell count in these was higher than the allowed detection limits of S. aureus according to the Turkish Food Codex (Codex 2011). Previously, higher numbers of coagulase positive S. aureus were reported from different regions in traditional cheese samples (Rola et al. 2016; Saka and Terzi Gulel 2018). Therefore, more attention should be paid to the milk quality by practicing more hygiene to avoid the occurrence of possible problems associated with these traditional cheese samples. Producers can select longer ripening periods recognized in the industry, such as 6 months and 1 year, as previously discussed (Öner et al. 2006).
The yeast and mold numbers in cheese samples were observed to be between 2.1–5.9 log CFU/g and 1.2–6.2 log CFU/g at days 1 and 90, respectively. These numbers were similar to previous observations (Macedo et al. 1995; Öner et al. 2006), and in general, no decrease in the yeast and mold counts during the ripening period was observed, which was in agreement with previous findings (Öner et al. 2006). The non-inhibition of the yeasts and molds in white cheese samples was associated with their potential to metabolize lactic acid, and they might also contribute to the ripening of cheese (Macedo et al. 1995). Nonetheless, in terms of white cheese, these high numbers of cells are not acceptable according to the Turkish Food Codex (Codex 2011).
Identification of LAB and nsLAB
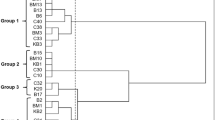
During the time of ripening, a slightly increase in the count of the LAB was observed. At the first sampling time, a mean count of 7 log CFU/g for Lactobacillus spp. and 9 log CFU/g for Lactococcus spp. was obtained. Further into the ripening phase, the numbers increased slightly and the highest counts of all microbial groups (> 10 log CFU/g) were reached at 90 days of ripening (Fig. 2). Our result was also in accordance with previous studies (Öner et al. 2006; Dertli et al. 2012). The numbers on the two different media (M17 and MRS agar) at the same sampling time were generally similar. After selecting the colonies according to phenotypic characteristics on both agars M17 and MRS, a total of 375 isolates (three to five isolates for each phenotype) were obtained from the different cheese samples during different ripening periods. We selected one isolate for each typical phenotype for further cultural characterization. Additionally, we selected a total of 51 isolates (one isolate for each typical phenotype) for further phenotypic identification (Table 2). The genotypic identification of the selected isolates (n = 32) by sequence analysis of the 16S rRNA gene revealed the presence of eight Lactococcus (Lc.) lactis, two Latilactobacillus (Lt.) curvatus and each one isolate of Lactobacillus (Lb.) casei and Lb. plantarum, eight Enterococcus (E.) durans, four E. faecalis, one E. faecium, five Streptococcus (St.) macedonicus, and one Weissella (W.) paramesenteroides, collected from traditional white cheese samples. Figure 3 demonstrates the MEGA X alignments of the 16S rRNA partial gene sequences of selected distinct LAB isolates (n = 32). This reveals their phylogenetic relationship, which resulted in the formation of different subgroups according to their species identification.
So far, several LAB species, especially enterococci, were reported to be present in the natural microflora of white cheese samples (Hayaloglu et al. 2002; İspirli et al. 2017; Uymaz et al. 2019). Importantly, all isolates of enterococci obtained in this study were still present in white cheese samples at day 90 of the ripening period. These observations indicate that enterococci may play a role as nsLAB in Kırklareli white cheese. Similar to our findings, it has been previously reported that E. durans and E. faecalis were dominant species in Turkish white cheese (İspirli et al. 2017). Another species isolated in the present study was St. macedonicus, and previous studies reported the presence of this species in different cheeses including Turkish white cheese (Lombardi et al. 2004; Ozteber and Başbülbül 2017; Uymaz et al. 2019). St. macedonicus was suggested to play a role in the formation of the characteristic flavor of some cheese types (Gobbetti et al. 2015) as a potential nsLAB. It should be noted that unlike previous findings, a lower level of St. macedonicus was present in Turkish white cheese, indicating that this bacteria may also be present in moderate amounts in Turkish white cheese (Uymaz et al. 2019). Compared to the enterococci isolates, St. macedonicus was present in the white cheese samples up until day 15 of ripening, which was also the case for the isolate W. paramesenteroides #11–15-A. Previous reports also confirmed the presence of Weissella species in several cheeses, including Ezine cheese (Gerasi et al. 2003; Uymaz et al. 2019).
Furthermore, within important LAB species for cheese manufacturing as starter cultures, eight isolates of Lc. lactis, two isolates of Lt. curvatus, and each one isolate of Lb. plantarum and Lb. casei were obtained from the different samples in the present study. Similar to our findings, it has been previously reported that these species were dominant starter cultures in Turkish white cheese (Ertürkmen and Öner 2015; İspirli et al. 2017). Two distinct isolates of Lt. curvatus (#12–30-B and #8–30-D) were isolated. Previously, Lt. curvatus as nsLAB and supplementary culture (Gobbetti et al. 2015) have been shown to be present in the microflora of different cheese types as well as other dairy products (Antonsson et al. 2003; Ozteber and Başbülbül 2017). The main LAB species isolated in this study were also Lc. lactis, confirming previous observations (Ertürkmen and Öner 2015; İspirli et al. 2017). Overall, the results of this study showed the presence of different LAB isolates, all of which could be associated with ripening of different cheese samples.
Technological characteristics of LAB
A rich microbial diversity in LAB was observed in Kırklareli white brined cheese samples, which can originate from low pasteurization standards, as temperatures above 65 °C were not reached during the cheese production. These LAB constitute the natural starter microflora of white cheese, as no starter cultures are used in the production of Kırklareli white cheese. To understand the starter potential of LAB from white cheese, several properties of selected isolates were tested, as their moderate acid-forming ability and proteolytic activity are crucial for their use as starter cultures during white cheese production (Settanni and Moschetti 2010).
In terms of acid production, a rapid pH decline is essential to achieve adequate coagulation, curd firmness, and control of bacterial pathogen growth. The ΔpH value of the isolates, where a value of < 1 is considered low, between 1 and 1.5 is considered medium, and greater than 1.5 is considered to have a high acid-forming level (Bradley et al. 1992), was assessed. All isolates showed a low acidogenic activity in skimmed milk, with a pH decrease (ΔpH6) after 6-h incubation at 30 °C ranging from 0.00 to 0.96 pH units. Generally, acid production levels were at an intermediate level for the entire 24-h incubation period ranging from 0.00 to 2.12 pH units, and the highest acidification activity was observed in Lc. lactis isolate #7–30-D, suggesting their potential as starter lactic acid bacteria (LAB) (Marshall 1992; Settanni and Moschetti 2010). In this context, the acidification activity of Lc. and Lb. isolates was determined by isolate-specific characteristics, indicating their potential use as starter and/or adjunct cultures to prevent defective fermentations. The results are presented in Table 2.
Another important characteristic of the cheese isolates was the proteolytic activity, which ranged from 34.05 to 76.45%, the expression of which was determined by isolate-specific characteristics. None of the isolates was positive for the production of H2S. Ammar et al. (2018) categorized the proteolytic activity values of the isolates as strong, moderate, and low, with proteolytic activity values of 100–200, 50–100, and less than 50 μg tyrosine in milliliters, respectively. The results of the present study showed that all found isolates had moderate proteolytic activity except isolates E. faecalis #P13 12–90-B and St. macedonicus #P18 2–15-C, which had low levels of proteolytic activity. In general, isolates with moderate proteolytic activity should be favored for white cheese production in order to avoid the development of bitterness during ripening.
As shown in Table 3, all isolates were sensitive to most antibiotics tested in this study, and 25 different antibiotic profiles (AP1 to AP25) were observed in terms of the degree of inhibition depending on isolate-specific conditions and the antibiotics tested. For example, all isolates were strongly inhibited by ampicillin, whereas the zones of inhibition were generally lower for kanamycin and streptomycin. These two antibiotics have also been shown to be among the antibiotics to which LAB can exhibit high levels of resistance (Pesavento et al. 2014; İspirli et al. 2015). Importantly, the presence of vancomycin-resistant was noteworthy in a few isolates within the antibiotic profiles AP1, AP2, and AP3 in this study. It is worth mentioning that previous reports have documented certain isolates of enterococci derived from human feces and cheese as vancomycin-resistant (İspirli et al. 2015, 2017). Moreover, no antibiotic resistance was detected in various St. macedonicus isolates isolated in this study. The results presented here are consistent with previous observations demonstrating the susceptibility of St. macedonicus isolates (Lombardi et al. 2004). Similar to previous reports of low antibiotic resistance in W. paramesenteroides (Jeong and Lee 2015), the cheese isolate W. paramesenteroides #P19 11–15-A was not found to be resistant to the antibiotics tested. Despite the promising technological properties observed, the isolates of Enterococcus spp., St. macedonicus, and W. paramesenteroides found in the current study cannot be considered for incorporation into cheese production. This is because this species does not have qualified presumption of safety status due to being among the leading causes of community- and nosocomial infections (EFSA 2007). Furthermore, in contrast to previous studies showing resistance of the Lt. curvatus isolate to kanamycin and streptomycin (Shazali et al. 2014), the two Lt. curvatus isolates from white cheese samples were sensitive to all tested antibiotics, including kanamycin and streptomycin. Overall, the results of this study showed that the selected Lc. lactis and Lb. plantarum and Lb. casei isolates within both antibiotic susceptibility profiles AP24 and AP25 (Table 3) were not found to be resistant to antibiotics, which may be a positive feature for their use in industrial production of these cheeses.
Another important characteristic of LAB cultures from fermented food products is their antibacterial activity. The antibacterial activities of different LAB isolates were tested against important food pathogens B. cereus FMC 19, E. coli ATCC 25922, L. monocytogenes RSKK 472, S. typhimurium NRRLE 4463, and S. aureus ATCC 28213 (Table 4). In general, the antibacterial activity of the isolates was low, as only three of 18 isolates showed antibacterial activity (Table 4). The highest antibacterial activity was observed for the isolate E. durans #P3 5–1-C, as this isolate strongly inhibited B. cereus FMC 19, E. coli ATCC 25922, and L. monocytogenes RSKK 472. The isolate E. durans #P4 9–90-B similarly inhibits E. coli ATCC 25922 and L. monocytogenes RSKK 472, although this isolate was not effective against B. cereus FMC 19. The last isolate to show antibacterial activity in this study was Lt. curvatus #12–30-B, which was only effective against L. monocytogenes RSKK 472. Apart from these three isolates, no other 33 isolates including Lc. lactis and Lb. casei and Lb. plantarum showed antibacterial activity, suggesting that isolate-specific characteristics determine antibacterial activity, which may be due to the production of antibacterial substances such as bacteriocins (İspirli et al. 2017). Studies testing the genotypic and phenotypic characteristics of the bacteriocin production abilities of these isolates are still ongoing.
Conclusion
This study characterized the physicochemical and microbiological properties of traditionally produced Kırklareli white brined cheese, and the nsLAB/sLAB profile was determined during ripening. As potential starter cultures, Lc. lactis dominated the bacterial profile in the cheese samples, followed to a lesser extent by Lb. casei and Lb. plantarum. Worthy of mention, no antibiotic resistance was observed for any of these isolates. Moderate levels of acidifying and proteolytic activity were observed in all isolates, confirming their potential as starter culture in traditional white cheese production in the Kırklareli region. The results presented here allow a first conclusion to be drawn about the suitability of these isolated properties in cheese production, thus forming the basis for further investigations. The presence of these Lc. lactis, Lb. casei, and Lb. plantarum isolates as potential adjunct cultures in white cheese should be further investigated. Indeed, studies on this topic were already initiated with some of these isolates.
References
Albayrak ÇB, Duran M (2021) Isolation and characterization of aroma producing lactic acid bacteria from artisanal white cheese for multifunctional properties. LWT - Food Sci Technol 150:112053. https://doi.org/10.1016/J.LWT.2021.112053
Ammar ET, El-Shazly A, Zalma S, El-Sharoud W (2018) Isolation, technological characterization and safety assessment of potential adjunct cultures of lactic acid bacteria. J Food Dairy Sci 9:19–29. https://doi.org/10.21608/jfds.2018.35153
Antonsson M, Molin G, Ardö Y (2003) Lactobacillus strains isolated from Danbo cheese as adjunct cultures in a cheese model system. Int J Food Microbiol 85:159–169. https://doi.org/10.1016/s0168-1605(02)00536-6
Beuchat LR, Ryu JH (1997) Produce handling and processing practices. Emerg Infect Dis 3:459–465. https://doi.org/10.3201/eid0304.970407
Bradley RL, Arnold E, Barbano DM, Semerad RG, Smith DE, Vines BK (1992) Chemical and physical methods. In: Marshall RT (eds) Stardard Methods for the Examination of Dairy Product. American Public Health Association, Washington, DC, pp 433-531. https://doi.org/10.2105/9780875530024
Citti J, Sandine W, Elliker P (1963) Some observations on the hull method for measurement of proteolysis in milk. J Dairy Sci 46:337. https://doi.org/10.3168/jds.S0022-0302(63)89042-6
Codex (2011) Turkish Food Codex Regulation on contaminants. Official Gazette No. 28157, Ankara, 29 December 2011. Available at: https://www.resmigazete.gov.tr/eskiler/2023/11/20231105-1.htm. Accessed 04.12.2023
Çakmakçı S, Kurt A (1993) Effect of salt amount of brine and ripening period, CaCI2 and lecithin addition made on white pickled cheese quality. Gıda 18:21–28. Available at https://dergipark.org.tr/tr/pub/gida/issue/6965/92855. Accessed 04 Dec 2023
Dertli E, Mercan E, Arici M, Yilmaz MT, Sağdiç O (2016) Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT - Food Sci Technol 71:116–124. https://doi.org/10.1016/j.lwt.2016.03.030
Dağdemir E, Celik Ş, Özdemir S (2003) The effects of some starter cultures on the properties of Turkish white cheese. Int J Dairy Technol 4:215–218. https://doi.org/10.1046/j.1471-0307.2003.00103.x
Dertli E, Sert D, Akin N (2012) The effects of carbon dioxide addition to cheese milk on the microbiological properties of Turkish white brined cheese. Int J Dairy Technol 65:387–392. https://doi.org/10.1111/j.1471-0307.2012.00843.x
EFSA, European Food Safety Authority (2007) Introduction of a qualified presumption of safety (QPS) approach for assessment of selected microorganisms referred to EFSA - opinion of the scientific committee. EFSA J 587:1–16. https://doi.org/10.2903/j.efsa.2007.587
EFSA, European Food Safety Authority (2018) Risk assessment of antimicrobial resistance along the food chain through culture-independent methodologies. EFSA J 16(S1):e160811. https://doi.org/10.2903/j.efsa.2018.e160811
Ertürkmen P, Öner Z (2015) Beyaz peynir örneklerinden izole edilen laktik asit bakterilerinin başlatıcı (starter) kültür özelliklerinin biyokimyasal yöntemlerle belirlenmesi. SD University J Nat Appl Sci 19:9–16. https://doi.org/10.19113/sdufbed.25545
Feng P, Weagant SD, Grant MA, Burkhardt W (2002) Chapter 4. Enumeration of Escherichia coli and the Coliform Bacteria. In: Food and Drug Administration (FDA), Bacteriological Analytical Manual Online, 8th Edition, Silver Spring, Berlin, 1998
Gerasi E, Litopoulou-Tzanetaki E, Tzanetakis N (2003) Microbiological study of Manura, a hard cheese made from raw ovine milk in the Greek island Sifnos. Int J Dairy Technol 56:117–122. https://doi.org/10.1046/j.1471-0307.2003.00085.x
Gobbetti M, De Angelis M, Di Cagno R, Mancini L, Fox P (2015) Pros and cons for using non-starter lactic acid bacteria (NSLAB) as secondary/adjunct starters for cheese ripening. Trends Food Sci Technol 45:167–178. https://doi.org/10.1016/j.tifs.2015.07.016
Grujović MZ, Mladenović KG, Semedo-Lemsaddek T, Laranjo M, Stefanović OD, Kocić-Tanackov SD (2021) Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: potential use as starters or probiotics. Compr Rev Food Sci Food Saf 21:1537–1567. https://doi.org/10.1111/1541-4337.12897
Hayaloglu A, Guven M, Fox PF (2002) Microbiological, biochemical and technological properties of Turkish white cheese ‘Beyaz Peynir.’ Int Dairy J 12:635–648. https://doi.org/10.1016/S0958-6946(02)00055-9
Hayaloglu AA, Guven M, Fox PF, McSweeney PLH (2005) Influence of starters on chemical, biochemical, and sensory changes in Turkish white-brined cheese during ripening. J Dairy Sci 88:3460–3474. https://doi.org/10.3168/jds.S0022-0302(05)73030-7
ISO (2005) International Standards Organisation, Microbiology of the food chain Horizontal method for the detection and enumeration of Enterobacteriaceae Part 2: Colony-count technique. ISO 21528, 2nd ed, International Standards Organisation, Geneva, Switzerland
İspirli H, Demirbaş F, Dertli E (2015) Characterization of functional properties of Enterococcus faecium strains isolated from human gut. Can J Microbiol 61:861–870. https://doi.org/10.1139/cjm-2015-0446
İspirli H, Demirbaş F, Dertli E (2017) Characterization of functional properties of Enterococcus spp. isolated from Turkish white cheese. LW - TFood Sci Technol 75:358–365. https://doi.org/10.1016/j.lwt.2016.09.010
Jeong DW, Lee JH (2015) Antibiotic resistance, hemolysis and biogenic amine production assessments of Leuconostoc and Weissella isolates for kimchi starter development. LWT - Food Sci Tech 64:1078–1084. https://doi.org/10.1016/j.lwt.2015.07.031
Lee B, Simard R (1984) Evaluation of methods for detecting the production of H2S, volatile sulfides, and greening by lactobacilli. J Food Sci 49:981–983. https://doi.org/10.1111/j.1365-2621.1984.tb10374.x
Lombardi A, Gatti M, Rizzotti L, Torriani S, Andrighetto C, Giraffa G (2004) Characterization of Streptococcus macedonicus strains isolated from artisanal Italian raw milk cheeses. Int Dairy J 14:967–976. https://doi.org/10.1016/j.idairyj.2004.04.005
Macedo A, Malcata F, Hogg T (1995) Microbiological profile in Serra ewes’ cheese during ripening. J Appl Bacteriol 79:1–11. https://doi.org/10.1111/j.1365-2672.1995.tb03117.x
Marshall RT (1992) Standard methods for the examination of dairy products. American Public Health Association (USA), 16th ed. DNAL SF253.A5
Ozteber M, Başbülbül G (2017) Antibiotic resistance patterns of lactic acid bacteria isolated from different fermented milk products of Turkish origin. Microbiol Res J Int 20:1–13. https://doi.org/10.9734/MRJI/2017/33221
Öner Z, Karahan AG, Aloğlu H (2006) Changes in the microbiological and chemical characteristics of an artisanal Turkish white cheese during ripening. LWT - Food Sci Technol 39:449–454. https://doi.org/10.1016/j.lwt.2005.03.015
Öner Z, Sarıdağ AM (2019) The changes during maturation of the white cheese produced from goat milk. GIDA 44:523–533. https://doi.org/10.15237/gida.GD19048
Pesavento G, Calonico C, Ducci B, Magnanini A, Nostro AL (2014) Prevalence and antibiotic resistance of Enterococcus spp. isolated from retail cheese, ready-to-eat salads, ham, and raw meat. Food Microbiol 41:1–7. https://doi.org/10.1016/j.fm.2014.01.008
Rola JG, Czubkowska A, Korpysa-Dzirba W, Osek J (2016) Occurrence of Staphylococcus aureus on farms with small scale production of raw milk cheeses in Poland. Toxins 8:62. https://doi.org/10.3390/toxins8030062
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Saka E, Terzi Gulel G (2018) Detection of enterotoxin genes and methicillin-resistance in Staphylococcus aureus isolated from water buffalo milk and dairy products. J Food Sci 83:1716–1722. https://doi.org/10.1111/1750-3841.14172
Sarantinopoulos P, Andrighetto C, Georgalaki MD, Reac MC, Lombardib A, Coganc TM, Kalantzopoulosa G, Tsakalidou E (2001) Biochemical properties of Enterococci relevant to their technological performance. Int Dairy J 11:621–647. https://doi.org/10.1016/S0958-6946(01)00087-5
Settanni L, Moschetti G (2010) Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol 27:691–697. https://doi.org/10.1016/j.fm.2010.05.023
Shazali N, Foo HL, Loh TC, Choe DW, Rahim RA (2014) Prevalence of antibiotic resistance in lactic acid bacteria isolated from the faeces of broiler chicken in Malaysia. Gut Pathog 6:1. https://doi.org/10.1186/1757-4749-6-1
Tallent S, Hait J, Bennett WB, Lancette GA (2001) Chapter 12. Staphylococcus aureus. In: Food and Drug Administration (FDA), Bacteriological Analytical Manual Online, 8th Edition, Silver Spring, Berlin, 1998
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Temmerman R, Huys G, Swings J (2004) Identification of lactic acid bacteria: culture-dependent and culture-independent methods. Trends Food Sci Technol 15:348–359. https://doi.org/10.1016/j.tifs.2003.12.007
Terzić-Vidojević A, Veljović K, Tolinački M, Živković M, Lukić J, Lozo J, Golić N (2020) Diversity of non-starter lactic acid bacteria in autochthonous dairy products from Western Balkan countries-technological and probiotic properties. Food Res Int 136:109494. https://doi.org/10.1016/j.foodres.2020.109494
Tournas V, Stack ME, Mislivec PB, Koch HA, Bandler R (2001) Chapter 18. Yeasts, Molds and Mycotoxins. In: Food and Drug Administration (FDA), Bacteriological Analytical Manual Online, 8th Edition, Silver Spring, Berlin, 1998
Uğur E, Öner Z (2018) Investigation of the effect of the commercial Salmonella phage preparation Salmonella spp in Beyaz (White) Cheese. TURJAF 6:995–1001. https://doi.org/10.24925/turjaf.v6i8.995-1001.1828
Uymaz B, Akçelik N, Yüksel Z (2019) Physicochemical and microbiological characterization of protected designation of origin Ezine cheese: assessment of non-starter lactic acid bacterial diversity with antimicrobial activity. Food Sci Anim Resour 39:804. https://doi.org/10.5851/kosfa.2019.e71
Vlková E, Rada V, Popelářová P, Trojanová I, Killer J (2006) Antimicrobial susceptibility of bifidobacteria isolated from gastrointestinal tract of calves. Livest Sci 105:253–259. https://doi.org/10.1016/j.livsci.2006.04.011
Yucel N, Ulusoy H (2006) A Turkey survey of hygiene indicator bacteria and Yersinia enterocolitica in raw milk and cheese samples. Food Control 17:383–388. https://doi.org/10.1016/j.foodcont.2005.01.005
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by the Scientific Research projects of Kırklareli University with the project number KLUBAP-147.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Çetin, B., Usal, M., Aloğlu, H.Ş. et al. Characterization and technological functions of different lactic acid bacteria from traditionally produced Kırklareli white brined cheese during the ripening period. Folia Microbiol (2024). https://doi.org/10.1007/s12223-024-01141-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12223-024-01141-8