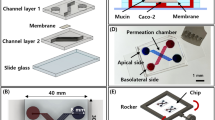
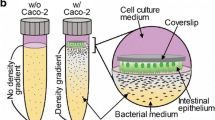
Abstract
Introduction
Gastrointestinal (GI) in vitro models have received lasting attention as an effective tool to model drug and nutrient absorption, study GI diseases, and design new drug delivery vehicles. A complete model of the GI epithelium should at a minimum include the two key functional components of the GI tract: mucus and the underlying epithelium. Mucus plays a key role in protecting and lubricating the GI tract, poses a barrier to orally administered therapies and pathogens, and serves as the microenvironment for the GI microbiome. These functions are reliant on the biophysical material properties of the mucus produced, including viscosity and pore size.
Methods
In this study, we generated in vitro models containing Caco-2 enterocyte-like cells and HT29-MTX goblet-like cells and determined the effects of coculture and mucus layer on epithelial permeability and biophysical properties of mucus using multiple particle tracking (MPT).
Results
We found that mucus height increased as the amount of HT29-MTX goblet-like cells increased. Additionally, we found that increasing the amount of HT29-MTX goblet-like cells within culture corresponded to an increase in mucus pore size and mucus microviscosity, measured using MPT. When compared to ex vivo mucus samples from mice and pigs, we found that a 90:10 ratio of Caco-2:HT29-MTX coculture displayed similar mucus pore size to porcine jejunum and that the mucus produced from 90:10 and 80:20 ratios of cells shared mechanical properties to porcine jejunum and ileum mucus.
Conclusions
GI coculture models are valuable tools in simulating the mucus barrier and can be utilized for a variety of applications including the study of GI diseases, food absorption, or therapeutic development.




Similar content being viewed by others
References
Aksoy, N., A. Corfield, and J. Sheehan. Preliminary study pointing out a significant alteration in the biochemical composition of MUC2 in colorectal mucinous carcinoma. Clin Biochem. 33:167–173, 2000. https://doi.org/10.1016/S0009-9120(00)00058-8.
Araújo, F., and B. Sarmento. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 458(1):128–134, 2013. https://doi.org/10.1016/j.ijpharm.2013.10.003.
Artursson, P., and J. Karlsson. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 175(3):880–885, 1991. https://doi.org/10.1016/0006-291x(91)91647-u.
Artursson, P., K. Palm, and K. Luthman. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46(1–3):27–43, 2001. https://doi.org/10.1016/s0169-409x(00)00128-9.
Atuma, C., V. Strugala, A. Allen, and L. Holm. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastroint. Liver Physiol. 280(5):G922–G929, 2001. https://doi.org/10.1152/ajpgi.2001.280.5.G922.
Atuma, C., V. Strugala, A. Allen, and L. Holm. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol.-Gastrointest. Liver Physiol. 280(5):922–929, 2001.
Bajka, B. H., N. M. Rigby, K. L. Cross, A. Macierzanka, and A. R. Mackie. The influence of small intestinal mucus structure on particle transport ex vivo. Coll. Surf. B Biointerfaces. 135:73–80, 2015. https://doi.org/10.1016/j.colsurfb.2015.07.038.
Bansil, R., and B. S. Turner. The biology of mucus: composition, synthesis and organization. Adv. Drug Deliv. Rev. 124:3–15, 2018.
Beterams, A., K. De Paepe, L. Maes, et al. Versatile human in vitro triple coculture model coincubated with adhered gut microbes reproducibly mimics pro-inflammatory host-microbe interactions in the colon. FASEB J. 35(12):e21992, 2021. https://doi.org/10.1096/fj.202101135R.
Briske-Anderson, M. J., J. W. Finley, and S. M. Newman. The influence of culture time and passage number on the morphological and physiological development of Caco-2 cells. Proc. Soc. Exp. Biol. Med. 214(3):248–257, 1997. https://doi.org/10.3181/00379727-214-44093.
Camilleri, M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 68(8):1516–1526, 2019. https://doi.org/10.1136/gutjnl-2019-318427.
Chen, X. M., I. Elisia, and D. D. Kitts. Defining conditions for the co-culture of Caco-2 and HT29-MTX cells using Taguchi design. J. Pharmacol. Toxicol. Methods. 61(3):334–342, 2010. https://doi.org/10.1016/j.vascn.2010.02.004.
Cone, R. A. Chapter 4 - Mucus. In: Mucosal Immunology (Third Edition), edited by J. Mestecky, M. E. Lamm, J. R. McGhee, J. Bienenstock, L. Mayer, and W. Strober. Cambridge: Academic Press, 2005, pp. 49–72.
Cone, R. A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 61(2):75–85, 2009. https://doi.org/10.1016/j.addr.2008.09.008.
Cornick, S., A. Tawiah, and K. Chadee. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 3(1–2):e982426–e982426, 2015. https://doi.org/10.4161/21688370.2014.982426.
Crocker, J. C., and D. G. Grier. Methods of digital video microscopy for colloidal studies. J. Coll. Interface Sci. 179(1):298–310, 1996.
Duncan, G. A., J. Jung, A. Joseph, et al. Microstructural alterations of sputum in cystic fibrosis lung disease. JCI Insight. 1(18):e88198–e88198, 2016. https://doi.org/10.1172/jci.insight.88198.
Duncan, G. A., J. Jung, A. Joseph, et al. Microstructural alterations of sputum in cystic fibrosis lung disease. JCI Insight. 2016. https://doi.org/10.1172/jci.insight.88198.
Engevik, A. C. Using microfluidics to model mucus. Cell. Mol. Gastroenterol. Hepatol. 9(3):551–552, 2020. https://doi.org/10.1016/j.jcmgh.2019.12.003.
Engle, M. J., G. S. Goetz, and D. H. Alpers. Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J. Cell Physiol. 174(3):362–369, 1998. https://doi.org/10.1002/(sici)1097-4652(199803)174:3%3c362::aid-jcp10%3e3.0.co;2-b.
Ensign, L. M., A. Henning, C. S. Schneider, et al. Ex vivo characterization of particle transport in mucus secretions coating freshly excised mucosal tissues. Mol. Pharm. 10(6):2176–2182, 2013. https://doi.org/10.1021/mp400087y.
Ermund, A., J. K. Gustafsson, G. C. Hansson, and Å. V. Keita. Mucus properties and goblet cell quantification in mouse, rat and human ileal Peyer’s patches. PLoS ONE.8(12):e83688, 2013.
Ferraretto, A., M. Bottani, P. de Luca, et al. Morphofunctional properties of a differentiated Caco2/HT-29 co-culture as an in vitro model of human intestinal epithelium. Biosci. Rep. 38(2):1497, 2018. https://doi.org/10.1042/bsr20171497.
Forstner, J. Intestinal mucins in health and disease. Digestion. 17(3):234–263, 1978.
Gagnon, M., A. Zihler Berner, N. Chervet, C. Chassard, and C. Lacroix. Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J. Microbiol. Methods. 94(3):274–279, 2013. https://doi.org/10.1016/j.mimet.2013.06.027.
Georgiades, P., P. D. Pudney, D. J. Thornton, and T. A. Waigh. Particle tracking microrheology of purified gastrointestinal mucins. Biopolymers. 101(4):366–377, 2014.
Gupta, V., N. Doshi, and S. Mitragotri. Permeation of insulin, calcitonin and exenatide across Caco-2 monolayers: measurement using a rapid, 3-day system. PLoS ONE. 8(2):e57136–e57136, 2013. https://doi.org/10.1371/journal.pone.0057136.
Hansson, G. C. Mucins and the microbiome. Annu. Rev. Biochem. 89:769–793, 2020.
Hill, D. B., P. A. Vasquez, J. Mellnik, et al. A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS ONE.9(2):e87681, 2014.
Howard, R. L., M. Markovetz, Y. Wang, et al. Biochemical and rheological analysis of human colonic culture mucus reveals similarity to gut mucus. Biophys. J. 120(23):5384–5394, 2021. https://doi.org/10.1016/j.bpj.2021.10.024.
Johansson, M. E., and G. C. Hansson. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16(10):639–649, 2016.
Johansson, M. E. V., J. M. H. Larsson, and G. C. Hansson. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl. Acad. Sci. 108(Supplement 1):4659, 2011. https://doi.org/10.1073/pnas.1006451107.
Johansson, M. E., M. Phillipson, J. Petersson, A. Velcich, L. Holm, and G. C. Hansson. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. 105(39):15064–15069, 2008.
Karam, S. M. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 4:D286–D298, 1999. https://doi.org/10.2741/karam.
Kernéis, S., A. Bogdanova, J.-P. Kraehenbuhl, and E. Pringault. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 277(5328):949–952, 1997. https://doi.org/10.1126/science.277.5328.949.
Krupa, L., B. Bajka, R. Staroń, et al. Comparing the permeability of human and porcine small intestinal mucus for particle transport studies. Sci. Rep. 10(1):20290, 2020. https://doi.org/10.1038/s41598-020-77129-4.
Lai, S. K., Y.-Y. Wang, and J. Hanes. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61(2):158–171, 2009. https://doi.org/10.1016/j.addr.2008.11.002.
Lai, S. K., Y.-Y. Wang, D. Wirtz, and J. Hanes. Micro- and macrorheology of mucus. Adv. Drug Deliv. Rev. 61(2):86–100, 2009. https://doi.org/10.1016/j.addr.2008.09.012.
Lea, T., et al. Caco-2 Cell Line. In: The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models, edited by K. Verhoeckx, P. Cotter, and I. López-Expósito, et al. Cham: Springer International Publishing, 2015, pp. 103–111.
Leal, J., H. D. C. Smyth, and D. Ghosh. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 532(1):555–572, 2017. https://doi.org/10.1016/j.ijpharm.2017.09.018.
Lesuffleur, T., N. Porchet, J.-P. Aubert, et al. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 106(3):771–783, 1993.
Lock, J. Y., T. L. Carlson, and R. L. Carrier. Mucus models to evaluate the diffusion of drugs and particles. Adv. Drug Deliv. Rev. 124:34–49, 2018. https://doi.org/10.1016/j.addr.2017.11.001.
Lock, J., T. Carlson, C.-M. Wang, A. Chen, and R. Carrier. Acute exposure to commonly ingested emulsifiers alters intestinal mucus structure and transport properties. Sci. Rep. 2018. https://doi.org/10.1038/s41598-018-27957-2.
Lozoya-Agullo, I., F. Araújo, I. González-Álvarez, et al. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture. Mol. Pharm. 14(4):1264–1270, 2017. https://doi.org/10.1021/acs.molpharmaceut.6b01165.
Macierzanka, A., A. R. Mackie, and L. Krupa. Permeability of the small intestinal mucus for physiologically relevant studies: impact of mucus location and ex vivo treatment. Sci. Rep. 9(1):17516, 2019. https://doi.org/10.1038/s41598-019-53933-5.
Mahler, G. J., M. L. Shuler, and R. P. Glahn. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J. Nutr. Biochem. 20(7):494–502, 2009. https://doi.org/10.1016/j.jnutbio.2008.05.006.
Maisel, K., M. Reddy, Q. Xu, et al. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine (London, England). 11(11):1337–1343, 2016. https://doi.org/10.2217/nnm-2016-0047.
Mason, T. G. Estimating the viscoelastic moduli of complex fluids using the generalized Stokes-Einstein equation. Rheologica Acta. 39(4):371–378, 2000.
Mason, T. G., and D. A. Weitz. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys. Rev. Lett. 74(7):1250, 1995.
Nance, E. A., G. F. Woodworth, K. A. Sailor, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 4(149):149ra119, 2012. https://doi.org/10.1126/scitranslmed.3003594.
Navabi, N., M. A. McGuckin, and S. K. Lindén. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS ONE.8(7):e68761, 2013. https://doi.org/10.1371/journal.pone.0068761.
Pan, F., L. Han, Y. Zhang, Y. Yu, and J. Liu. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int. J. Food Sci. Nutr. 66(6):680–685, 2015. https://doi.org/10.3109/09637486.2015.1077792.
Paone, P., and P. D. Cani. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 69(12):2232, 2020. https://doi.org/10.1136/gutjnl-2020-322260.
Pontier, C., J. Pachot, R. Botham, B. Lenfant, and P. Arnaud. HT29-MTX and Caco-2/TC7 monolayers as predictive models for human intestinal absorption: role of the mucus layer. J. Pharm. Sci. 90(10):1608–1619, 2001. https://doi.org/10.1002/jps.1111.
Srinivasan, B., A. R. Kolli, M. B. Esch, H. E. Abaci, M. L. Shuler, and J. J. Hickman. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 20(2):107–126, 2015. https://doi.org/10.1177/2211068214561025.
Swidsinski, A., Y. Dörffel, V. Loening-Baucke, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet Original Research. Front. Microbiol. 8:225, 2017. https://doi.org/10.3389/fmicb.2017.01141.
Ude, V. C., D. M. Brown, V. Stone, and H. J. Johnston. Using 3D gastrointestinal tract in vitro models with microfold cells and mucus secreting ability to assess the hazard of copper oxide nanomaterials. J. Nanobiotechnol. 17(1):70, 2019. https://doi.org/10.1186/s12951-019-0503-1.
Wu, S., M. Wu, D. Tian, L. Qiu, and T. Li. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ. Toxicol. 35(4):495–506, 2020. https://doi.org/10.1002/tox.22885.
Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man–fact or myth. Pharm. Res. 14(6):763–766, 1997. https://doi.org/10.1023/a:1012102522787.
Yildiz, H. M., C. A. McKelvey, P. J. Marsac, and R. L. Carrier. Size selectivity of intestinal mucus to diffusing particulates is dependent on surface chemistry and exposure to lipids. J Drug Target. 23(7–8):768–774, 2015. https://doi.org/10.3109/1061186X.2015.1086359.
Acknowledgments
We would like to thank the UMD Bioworkshop for core facility usage and Michele Kaluzienski for assisting with manuscript preparation. This work was funded by the University of Maryland New Directions Fund and Faculty-Student Research Award (KM) and the AHA Predoctoral Research Fellowship (JM).
Conflict of Interest
The authors (JM, AS, and KM) have no conflict of interest to declare.
Ethical Approval
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McCright, J., Sinha, A. & Maisel, K. Generating an In Vitro Gut Model with Physiologically Relevant Biophysical Mucus Properties. Cel. Mol. Bioeng. 15, 479–491 (2022). https://doi.org/10.1007/s12195-022-00740-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-022-00740-0