Abstract
Post-thrombotic syndrome (PTS) is a common and potentially debilitating complication of deep vein thrombosis (DVT), affecting up to 50% of DVT patients. The consequence of this chronic condition includes reduced quality of life, increased use of the healthcare system and decreased productivity. The societal impact of this condition is projected to increase, given our ageing population and increased burden of thrombotic diseases. Despite significant recent advances in our understanding of PTS, many unanswered questions remain. Currently, there are few effective and proven options for established PTS; hence, the emphasis should be on instituting effective prevention to reduce the progression to PTS. Effective anticoagulation lowers the risk of PTS, with direct oral anticoagulants appearing to outperform vitamin-K antagonists. However, the evidence for elastic compression stockings and endovascular thrombolysis or thrombectomy techniques remains unclear. Accurate identification of individuals at high risk of developing PTS may also improve the targeting of preventative interventions. This review will examine the current body of evidence regarding PTS, with a focus on preventative strategies as well as novel biomarkers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Post-thrombotic syndrome (PTS) is a common condition that afflicts up to 30–50% of individuals following deep vein thrombosis (DVT) [1,2,3,4,5]. It can be diagnosed from 3 to 6 months after an episode of DVT [6,7,8]. Symptoms and signs of PTS overlap with primary venous insufficiency and include leg pain, heaviness, fatigue, swelling, skin discolouration, lipodermatosclerosis and venous ulcers in severe cases. Symptoms are typically exacerbated by standing or walking and can range from mild to severe in up to 15% [9].
PTS is a major cause of reduced quality of life (QOL) [10,11,12,13], comparable to that caused by diabetes or chronic lung disease [13]. The presence of PTS is the main determinant of general and venous disease-specific QOL at 2 years after DVT diagnosis [13] and its impact appears to be similar for proximal and distal DVT [10]. PTS also adds to the health economic costs of DVT [14]. A Canadian prospective study of acute DVT patients [15] found that PTS was associated with a 35–45% increase in costs to the patient and healthcare system, including 62% attributable to non-medical costs such as loss of productivity [15]. Despite this, PTS is under recognised by many physicians, further compounding the QOL impact for these patients. Given that the global incidence of DVT is 0.5–1.5/1000 population [16], the total societal impact of PTS is substantial and one that could potentially be mitigated with effective preventative strategies.
The purpose of this review is to appraise the current body of knowledge about PTS, with an emphasis on preventative strategies including novel biomarkers. The treatment modalities for PTS remain understudied and only modestly effective. As a result, more emphasis should be placed on preventing the development of PTS.
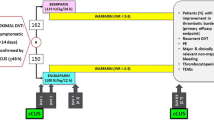
A systematic review of the literature was conducted to compile current evidence for the section on preventative treatments. The Medline (PubMed) database was searched on 5 July 2022, using the search strategy set out in the supplementary materials. The resulting search yielded 235 studies. Criteria used in identifying, screening and excluding studies are outlined in the PRISMA diagram (Fig. 1). The resulting 28 studies were compiled into three sections with anticoagulation in Table 2, elastic compression stockings in Table 3 and catheter-based early thrombus removal in Table 4.
Pathophysiology
The pathophysiology of PTS is incompletely understood, but is believed to result from venous hypertension caused by venous valvular damage and venous outflow obstruction from residual thrombosis and vessel wall fibrosis [17, 18] (Fig. 2). Venous hypertension and alterations of blood flow contributes to a chronic state of inflammation, by activating the endothelium and upregulating the expression of leucocyte adhesion molecules, causing release of pro-inflammatory cytokines and disrupting the glycocalyx [19,20,21,22]. This pro-inflammatory response and the consequences of the interaction of the thrombus with the vessel wall are likely integral to the pathogenesis of PTS [23]. However, due to the difficulties of collecting human deep vein tissues, our understanding of the molecular basis of PTS is mostly based on animal models. Recent human radiological studies showing thicker venous walls in PTS patients compared to acute DVT and normal controls support a role for venous vascular remodelling in the pathophysiology of PTS [24, 25].
Impaired fibrinolysis has been implicated in the development of PTS as residual thrombus is a known risk factor for PTS. However, measurements of individual components of the fibrinolytic system in PTS patients have shown conflicting results [26]. Besides its primary action to break down fibrin, plasmin(ogen) facilitates wound repair via a variety of plasminogen receptors, including those found on neutrophils and macrophages, which are the key cells involved in deep vein thrombosis resolution [27, 28]. Critical to this regenerative capacity is the ability of plasmin to directly activate metalloproteinases (MMP) from proMMPs, which act to degrade extracellular matrix and basement membrane components [29]. MMP-2 and MMP-9 have been shown to be increased following DVT [30, 31]. MMP-9 deleted mice showed increased vessel wall stiffness during thrombus resolution [32, 33]. Elevated PAI-1 leads to reduced MMP-2 and MMP-9 activity and vein wall fibrosis [34]. Venous thrombosis also causes the release of mediators including TGFb, IL-13 and MCP-1 which further promotes fibrosis [31, 35]. The fibrinolytic system could therefore be attractive targets for prevention of PTS. Despite a large number of candidate compounds, none have entered human trials to date [36]. The complex interplay of the thrombotic, fibrinolytic and inflammatory systems, together with our incomplete understanding of PTS pathogenesis, adds to the difficulty in finding effective therapeutic targets in PTS.
Risk factors for PTS
The recognised risk factors for development of PTS are listed in Table 1. The most important of these include proximal location of DVT (especially femoral and iliac veins), recurrent DVT history and obesity [3,4,5, 37,38,39,40,41,42]. Pre-existing chronic venous disease [37] and the presence of venous insufficiency signs in the contralateral leg [3] are risk factors for development of PTS and raise the possibility that primary venous insufficiency and PTS may share mechanistic pathways. An association with female sex is shown in some studies [2, 37, 41], but not others [3,4,5, 38, 39]. Thrombophilia, however, is not a proven risk factor [37, 43].
Diagnosis
The diagnosis of PTS is predominantly based on clinical symptoms, and one of the challenges is the considerable overlap of symptoms between PTS, recurrent DVT and primary venous insufficiency [44]. To help standardise clinical PTS diagnosis, various diagnostic tools have been formulated, each with its strengths and drawbacks [45, 46] (Supplemental Tables S1-3). The Villalta score is currently the most widely used score in both clinical practice and research and has been found to have high inter-observer reliability [47]. It is endorsed by the International Society of Thrombosis and Haemostasis (ISTH) and American Heart Association (AHA) [48, 49]. However, there remains no gold standard adjunctive biomarker or diagnostic test for PTS which makes both the recognition and diagnosis of PTS subjective to symptom reporting and clinician judgement. Additionally, given that many DVT patients have concurrent chronic venous insufficiency [3], PTS may also represent the natural progression or acceleration of an underlying and pre-existing chronic venous disease process.
PTS symptoms can mimic those of acute DVT, and in patients with recurrent symptoms in the ipsilateral leg, compression duplex ultrasonography (CUS) may be unable to distinguish acute from chronic thrombus [50] and previous imaging may not always be available for comparison, particularly if follow-up care is fragmented. Magnetic resonance (MR) venography is more accurate than CUS, particularly in the iliac veins, and also permits more accurate detection of venous wall scaring and venous inflow assessment [51, 52]. Recently, magnetic resonance direct thrombus imaging (MRDTI) has been shown to be highly sensitive and specific in the diagnosis of recurrent DVT. This method is based on the detection of high signal in T1-weighted MRI from methaemoglobin within blood clots, which disappears completely after 6 months. In a prospective study of 305 patients [53] with suspected recurrent ipsilateral DVT, inconclusive diagnoses were reduced from 30% to < 1% using MRDTI, with only 2 patients (1.7%) developing VTE within 30 days. MRDTI does not require contrast, is quick to perform and has excellent reproducibility between observers and across centres; it has the potential to change practice in this challenging patient population. Other imaging findings that can support a PTS diagnosis include luminal stenosis or narrowing, fibrotic bands, venous reflux and the presence of collateral veins [50]. CUS, CT venography and/or MR venography each have respective roles, but is substantially influenced by experience and familiarity between centres.
Current preventative strategies
Prevention remains the mainstay of PTS management and involves a combination of effective anticoagulation, use of elastic compression stocking in certain scenarios as well as early identification of high-risk VTE which may benefit from early catheter-based thrombolysis or thrombectomy.
Anticoagulation
Effective anticoagulation is one of the most effective strategies to prevent PTS, through early thrombus resolution by preventing thrombus propagation, and therefore reducing valve damage and residual vein obstruction, two of the major causes of PTS [54]. However, the duration of anticoagulation to treat DVT is not associated with improved clinical outcome, which was demonstrated in the ExACT randomised controlled trial (RCT) [55]. However, the time in the therapeutic range is critical, with subtherapeutic anticoagulation with vitamin K antagonists (VKAs) shown to increase the risk of PTS in several studies. [3, 41, 56]. This finding raises important questions about the type of anticoagulation used, particularly in the era of direct oral anticoagulants (DOAC), where all but one study shows DOACs to be associated with reduced likelihood of PTS (Table 2), likely in part due to their stable anticoagulation effect. The study that showed no difference [57] was a Danish registry study that relied on the McDougall criteria, based on symptoms and signs in medical records which may not be generalisable to clinical practice. Similarly, studies suggests that LMWH compared to VKAs likely reduce the risk of PTS and improves vein re-canalisation [58,59,60,61], of which the benefit conferred by LMWH may be twofold—more stable anticoagulation effect and possible anti-inflammatory effects [62]. Taken together, the body of knowledge to date highlights that effective anticoagulation is pivotal to reduce the occurrence of PTS.
Elastic compression stockings (ECS)
The mechanism by which ECS exert their beneficial effects are not entirely known. Some of the proposed mechanisms include reduction in the venous hypertension, increased venous flow velocity, reduction in venous reflux and blood volume in legs due to the reduction in the vein diameter of major veins and improving lymphatic drainage due to increase in tissue pressure [63, 64].
The evidence for ECS is mixed with several randomised controlled studies (RCT) comparing ECS to no stockings demonstrating around 50% reduction in PTS incidence (see Table 3). However, the largest placebo-stocking controlled trial of 804 patients (‘SOX’ study) [65] failed to show any interventional effect. In this study, PTS developed in 14.2% of the active ECS group vs 12.7% in the placebo group (HR 1.13, 95% CI 0.73–1.76, p = 0.58). However, one reason for the lack of efficacy could be due to the substantially lower compliance rate in this study compared to other RCTs. Barriers to optimal ECS usage may include discomfort, cost and difficulty putting on the stockings. Studies have examined the effect of modifications to the standard ECS regimen to improve compliance and found non-inferior results with reduced compression strength stockings (25 mmHg instead of 35 mmHg) [66], reduced ECS duration to 12 months [67] and an individualised tailored regimen based on Villalta score [68]. These strategies may be adopted in clinical practice to improve compliance with ECS. Nonetheless, the results of the SOX study have proved influential and have resulted in recent VTE guidelines recommending ECS for reduction of symptoms rather than direct prevention of PTS [69, 70].
Catheter-based early thrombus removal
Early thrombus removal can rapidly improve venous circulation in symptomatic iliofemoral DVT. It may also prevent PTS development in this high-risk group by removing thrombus at an early stage when thrombolysis or thrombectomy techniques are more likely to be effective. Endovascular techniques include catheter-directed thrombolysis (CDT) where thrombolytics are administered through a multi-sidehole infusion catheter placed across the thrombosed venous segment and pharmacomechanical catheter-directed thrombolysis (PCDT) in which an endovascular device macerates or extracts the thrombus in conjunction with thrombolysis, thus reducing the dose and duration of thrombolysis. To date, however, the data for the use of these methods remain relatively mixed, with important questions still to be clarified.
Table 4 summarises the results of three high-quality randomised controlled trials of CDT/PCDT. The CaVenT study [71] randomised 209 patients with iliofemoral DVT to CDT or standard therapy with anticoagulation alone within 21 days of symptom onset. At two-year follow-up, a minor interventional effect was found (PTS rates 41.1% vs 55.6%, p = 0.047). The final 5-year CaVenT [72] results, however, demonstrated a more marked difference in the intervention group (PTS rates 43% vs 71%, p = 0.001). Major bleeding in CaVenT occurred in 2.7% of the CDT group vs none in the control group. The CAVA study [73] randomised 152 patients to receive ultrasound accelerated catheter-directed thrombolysis (UACDT) or standard therapy in iliofemoral DVT within 21 days of symptom onset. At 1 year, PTS rates were no different for both groups (OR 0.75, 95% CI 0.38–1.50, p = 0.42), although the final follow-up (median 39 months) demonstrated lower PTS rates in the interventional group (46.8% vs 69%, OR 0.40 95%CI 0.19–0.84, p = 0.01) [74]. Major bleeding occurred in 5% of the CDT group vs none in the control group.
The ATTRACT study [75] randomised 692 patients including both iliofemoral and femoropopliteal DVTs, to various PCDT techniques (including AngioJet or Trellis pharmacomechanical thrombectomy) against standard therapy. No differences in PTS rates were found at 24 months; however, there was a significant but modest reduction in moderate-to-severe PTS as a composite secondary post hoc outcome in the PCDT subgroup with acute iliofemoral DVT (see Table 4). Major bleeding occurred in 1.7% of PCDT vs 0.3% in the control group (RR 6.18, 95%CI 0.78–49.2, p = 0.049). Several potential causes for the weak effect sizes have been identified in post hoc analyses. PCDT may not be effective for femoropopliteal DVTs (43% of study patients) [76] and is less likely to benefit those over the age of 65 [75]. In addition, PCDT did not significantly reduce incidence of valvular reflux, despite reducing residual thrombus volume [77]. The optimal timing of intervention is also unclear, but it is generally accepted that early intervention derives the greatest benefit. Post hoc analysis of ATTRACT found that intervention within 4–8 days from symptom onset was associated with the greatest benefits in QOL and Villalta scores. Interestingly, no significant improvement was seen in those who received intervention at < 4 or > 8 days [78]. Long-term data from ATTRACT have not been released, but if made available, they may provide additional insights.
The current evidence for endovascular intervention remains strongest in patients with acute, proximal iliofemoral DVT where there are potential gains in reducing the incidence and severity of PTS. However, the choice of CDT or PCDT remains uncertain and ATTRACT has not provided a satisfactory answer in this regard, particularly considering the high consumable cost associated with mechanical thrombectomy and the higher risk of clinically major bleeding compared to anticoagulation alone. Nevertheless, this is an important and active area of research and has the potential to greatly improve outcomes in a high-risk group. There is a need for more tailored randomised clinical trials that address the findings and deficiencies of previous studies. This includes defining the best group to target to maximise interventional benefit, as well as designing studies to address the optimal timing of intervention and the most effective type of catheter thrombus removal technique as well as cost–benefit analysis of newer generation devices over CDT alone.
Other pharmacotherapy as preventative agents
Several drugs with anti-inflammatory effects are candidates for prevention of post-thrombotic syndrome (PTS), but evidence to date is limited. Statins have been found to reduce venous thromboembolism (VTE) rates in a large RCT (HR 0.61, 95%CI 0.37–0.86, p = 0.007) [79], but their impact on PTS prevention is uncertain. The recent SAVER pilot RCT [80] did not show any difference in PTS, including in the DVT subgroup although a larger trial is planned (ClinicalTrials.gov NCT04319627). Sulodexide, an orally bio-available glycosaminoglycan mixture, possesses antithrombotic, anti-inflammatory and endothelial protective effects without increasing the risk of bleeding [81]. Although widely used in Europe, it does not have approval from the US Food and Drug Administration (FDA) or Australian Therapeutic Goods Administration (TGA) [82]. The SURVET RCT [83] found that sulodexide after the initial anticoagulation phase of DVT reduced recurrence of VTE compared to placebo (adjusted HR 0.45, 95%CI 0.24–0.84; p = 0.01) without increasing bleeding. Its effectiveness in preventing PTS has not been well studied, with only an Italian registry study [84] showing reduced PTS rates at 5 years in the sulodexide group (12.17% vs 18.23% p < 0.05). Evidence is lacking on the efficacy of venoactive agents such as diosmin and rutosides in preventing PTS, despite their use to relieve symptoms of chronic venous insufficiency [85]. A small RCT investigating Diosmin 600 [86] found significantly lower PTS in the intervention group after 12 months following iliofemoral DVT. A larger RCT of micronised purified flavonoid fraction (MPFF) is in the planning phase [87]. The results of these and other randomised trials of promising compounds are anticipated with interest.
Treatment of established PTS
The treatment of established PTS is challenging and there have only been a few studies that have specifically explored treatment modalities in this setting. Current strategies are mostly extrapolated from chronic venous disease. This section discusses current limited evidence for the treatment of established PTS.
Compression therapy
While there is evidence to support the use of ECS in chronic venous disease to relieve symptoms and improve QOL [88], there is limited evidence for the use of ECS in established PTS. In an RCT including 35 patients with established PTS, ECS was not effective in reducing PTS symptoms compared to placebo [89], and a 2019 Cochrane review [90] of ECS also determined that there was very low-certainty evidence to support ECS or pneumatic devices in this setting, and that further studies were required.
Exercise
Exercise may improve venous blood return by improving calf and thigh pump function. A small RCT of 43 PTS patients [91] found no differences in Villalta scores after a 6-month exercise programme, but significant improvements were seen in VEINES-QOL, leg strength and quadriceps flexibility. Evidence from chronic venous disease [92, 93] suggests that exercise can improve symptoms, QOL and muscle function. Although the evidence is limited, exercise programmes are unlikely to be harmful, and the American Heart Association guidelines recommend a 6-month structured exercise programme in PTS patients who can tolerate it [49].
Pharmacotherapy
There is currently no proven pharmacological therapy for established PTS. Anticoagulation can prevent recurrent DVT, lowering the risk of further vasculature damage, but it has no direct effect on PTS. Venoactive agents are widely used for chronic venous disease in many parts of the world, but the level of evidence in this setting is low [85]. Few studies have specifically examined these compounds in PTS, and a 2018 Cochrane review of rutosides in PTS found no benefit [94]. Sulodexide can reduce symptoms of chronic venous disease [95], and several RCTs found that it improved venous ulcer healing rates [96]. However, its use is restricted due to the lack of FDA and TGA approval.
Endovascular intervention
Endovascular stenting of iliocaval occlusion is an attractive option in patients with severe PTS symptoms. This is an area of active research, but high-quality prospective evidence with long duration of follow-up is still lacking. In a 2015 meta-analysis [97] of retrospective and cohort studies, stent placement in those with chronic PTS and iliofemoral venous outflow obstruction (n = 1118) resulted in complete pain relief in 69.3% and 1-year primary patency rates of 79%. The major complication is stent thrombosis which has been reported in 13.7% within the initial 6 months, despite concurrent anticoagulation [98]. Re-intervention rates in instances of stent re-thrombosis or occlusion are high and have been reported to be between 15 and 40% within 4 years of stent placement [99]. This is of particular concern in younger patients who require lifelong follow-up and in whom the ramifications to QOL will be significantly impactful. More rigorous and longer-term patency and safety data of this promising intervention is required.
In those with combined superficial venous disease, treatment of superficial venous insufficiency is beneficial. Unfortunately, most patients with PTS will have deep venous valve incompetence and surgical options are limited in this setting. Deep venous valve reconstructions have low rates of long-term success in PTS patients [100]. Bioprosthetic venous valve implants may show promise and there is an ongoing prospective multi-centre study of the porcine VenoValve system (NCT04943172) [101].
Future directions in prevention of PTS
Patient selection appears to be useful for the targeting of preventative strategies and is likely to be key to ensuring maximum therapeutic benefit. Hence, the development of more effective diagnostic tools such as biomarkers and imaging techniques is important.
Predictive risk scores
Predictive scoring symptoms may help identify patients who are more likely to develop PTS as we move into the era of personalised medicine. Table 5 summarises the published predictive scoring schemes and their c-statistic scores. There are significant differences in the derivation cohorts of these models, however, which hamper their generalisability.
Imaging
A meta-analysis [102] of studies in DVT that correlated CUS findings with PTS found that residual vein thrombus detected between 6 weeks and 12 months is associated with an increased risk of PTS (OR 2.2 95%CI 1.8–2.6). PTS is also predicted by the presence of venous reflux (OR 1.3, 95% CI 1.03–1.7). However, there is significant methodological heterogeneity between studies, particularly in terms of ultrasound timing, CUS techniques and PTS scores. CUS may also be difficult to perform and interpret in patients who have obesity, oedema, unhealed ulcers, or pelvic or inguinal occlusions further hampering diagnostic sensitivity. A recent time-resolved MR venography study found that it could detect veno-lymphatic pathology in one-quarter of CUS negative cases [103]. Future research should focus on determining the best timing and nature of vasculature changes to best predict PTS. There may also be a role for more sensitive modalities like MR venography to fully characterise the changes in veins in patients at high risk of PTS.
Biomarkers
Identification of a suitable biomarker of PTS may augment diagnosis and improve clinical risk evaluation allowing for better targeting of preventative therapy. Several candidate biomarkers have been explored, the results of which are outlined in this next section.
Inflammatory markers and adhesion molecules
Several studies have found C-reactive protein (CRP) to be associated with development of PTS, during the subacute phase between 1 and 12 months after DVT [104]. Other cytokines including IL-6, IL-8, IL-10, TNF-a, RANTES, MCP-1 have shown inconsistent results [104, 105]. Adhesion molecules such as VCAM-1, ICAM-1, P-selectin and E-selectin are expressed by the activated endothelium and are required for leucocyte migration [106]. Of these, ICAM-1 shows the most consistent association with PTS [105, 107, 108], including in the BioSOX study [109] of 703 patients after a first proximal DVT.
Matrix metalloproteinases
Matrix metalloproteinases (MMP) are a group of proteolytic enzymes involved in the remodelling of the extracellular matrix and are regulated by tissue inhibitors of metalloproteinases (TIMPs). These play an important part of fibrosis formation post-VTE, which is a major contributor to PTS. In a study of 201 DVT patients [110], MMP-1 and MMP-8 were significantly higher at all time points up to 18 months in PTS patients compared to those without PTS, and TIMP-1 and TIMP-2 were found to be significantly lower at all time points.
Marker of thrombosis and fibrinolysis
Most studies have not found differences in levels of factor VIII, von Willebrand factor, fibrinogen, PAI-1, soluble thrombomodulin and peak thrombin in PTS patients compared to non-PTS patients [40, 111,112,113,114,115,116]. A systematic review [26] found only four studies showing a significant association between elevated D-dimer and PTS, two of which were conducted prior to commencing anticoagulation. Some have shown increased levels of tPA [113] and TAFI [112] in patients who developed PTS.
Global markers of fibrinolytic potential may be a more accurate reflection of overall fibrinolysis. We recently published pilot findings [116] from 190 DVT patients, 32.6% of whom developed PTS (median follow-up 643.5 days). Plasma was sampled from patients during anticoagulation (median 90 days after DVT diagnosis) and overall fibrin generation and fibrinolytic potential measured by the OHP assay. Despite being anticoagulated, patients who subsequently developed PTS showed significantly higher OCP, OHP (indicating increased fibrin generation potential) and reduced OFP% (indication reduced fibrinolytic potential) than those who did not develop PTS. Independent variables associated with PTS are displayed in Table 6. We found two biomarkers to be independent predictors of PTS: OHP and neutrophil/lymphocyte ratio (NLR). These results were incorporated into a predictive multivariate model with a good performance C-stat of 0.77.
The results from our study are novel as they utilise samples collected during the initial anticoagulation period, which removes the need to pause anticoagulation. Previous studies in PTS using CLT, a related global fibrinolytic assay, were performed after the cessation of anticoagulation [112] and found CLT to be correlated with Villalta score (r = 0.38), but not an independent predictor of PTS development. To the best of our knowledge, OHP has not been explored as a predictive biomarker for PTS. Our group has previously reported the findings of pilot studies demonstrating the predictive value of OHP in predicting VTE recurrence in anticoagulated patients [117], as well as oxygen requirement in COVID-19 patients from two waves of the COVID-19 pandemic [118, 119]. As a result, OHP may have a promising prognostic value in various thrombo-inflammatory and hypofibrinolytic diseases, such as PTS.
The neutrophil/lymphocyte ratio (NLR) is an emerging predictive biomarker in cardiovascular and inflammatory conditions [120]. While it has not been studied previously in PTS, elevated NLR has been associated with negative outcomes in venous thrombosis. In the large population-based Tromsø Study [121], NLR > 95th percentile was associated with increased risk of mortality following VTE (adjusted HR 2.13, 95%CI 1.26–3.58, p = 0.02). In a meta-analysis of 1424 patients with acute PE, NLR was associated with short-term mortality with negative predictive value 96.7% and positive predictive value 24.4%. NLR is also associated with increased risk of portal vein thrombosis in cirrhotic patients (HR 1.46, 95%CI 1.04–2.04, p = 0.028) [122]. These findings support the interconnected nature of the coagulation and inflammatory systems, and our identification of NLR as a predictor of PTS is consistent with this being the outcome of the thrombo-inflammatory state after DVT.
While our study is limited by relatively small numbers and heterogeneous inclusion criteria comprising proximal and distal DVTs, and those with multiple prior DVTs, we have shown that the addition of global coagulation biomarkers has the potential to improve identification of patients at higher risk of developing PTS. The use of these biomarkers should be considered in future randomised clinical studies to help identify the highest risk patients who are likely to benefit from more aggressive preventative interventions.
Conclusion
Despite recent advances in knowledge, PTS remains one of the most common, chronic and serious complications of DVT, with few effective treatment options. Identifying those who are at high risk of developing PTS, particularly the severe forms, is one of the most difficult challenges. This is the population that may benefit from more intensive preventative measures such as ECS, anticoagulation and invasive treatments. More research is also needed to determine the best target group for catheter-based interventions particularly from a QOL and cost–benefit perspective. Novel biomarkers may play a role in improving existing clinical predictive models, allowing for a more personalised approach.
References
Prandoni P, Kahn SR. Post-thrombotic syndrome: prevalence, prognostication and need for progress. Br J Haematol. 2009;145:286–95.
Ende-Verhaar YM, Tick LW, Klok FA, Huisman MV, Rosendaal FR, le Cessie S, et al. Post-thrombotic syndrome: short and long-term incidence and risk factors. Thromb Res. 2019;177:102–9.
Galanaud JP, Holcroft CA, Rodger MA, Kovacs MJ, Betancourt MT, Wells PS, et al. Predictors of post-thrombotic syndrome in a population with a first deep vein thrombosis and no primary venous insufficiency. J Thromb Haemost. 2013;11:474–80.
Kahn SR, Kearon C, Julian JA, Mackinnon B, Kovacs MJ, Wells P, et al. Predictors of the post-thrombotic syndrome during long-term treatment of proximal deep vein thrombosis. J Thromb Haemost. 2005;3:718–23.
Wik HS, Jacobsen AF, Sandvik L, Sandset PM. Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: a population-based, cross-sectional, case-control study. J Thromb Haemost. 2012;10:840–7.
Prandoni P, Noventa F, Quintavalla R, Bova C, Cosmi B, Siragusa S, et al. Thigh-length versus below-knee compression elastic stockings for prevention of the postthrombotic syndrome in patients with proximal-venous thrombosis: a randomized trial. Blood. 2012;119:1561–5.
Prandoni P, Lensing AWA, Prins MH, Frulla M, Marchiori A, Bernardi E, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized. Controlled trial. Ann Intern Med. 2004;141:249.
Brandjes DP, Büller HR, Heijboer H, Huisman MV, de Rijk M, Jagt H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349:759–62.
Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004;164:17–26.
Roberts LN, Patel RK, Donaldson N, Bonner L, Arya R. Post-thrombotic syndrome is an independent determinant of health-related quality of life following both first proximal and distal deep vein thrombosis. Haematologica. 2014;99:e41–3.
Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health-related quality of life after deep venous thrombosis. Arch Intern Med. 2002;162:1144–8.
Kahn SR, M’Lan CE, Lamping DL, Kurz X, Bérard A, Abenhaim L, et al. The influence of venous thromboembolism on quality of life and severity of chronic venous disease. J Thromb Haemost. 2004;2:2146–51.
Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–12.
Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10.
Guanella R, Ducruet T, Johri M, Miron M-J, Roussin A, Desmarais S, et al. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: a prospective evaluation. J Thromb Haemost. 2011;9:2397–405.
Wendelboe AM, Raskob GE. Global burden of thrombosis. Circ Res. 2016;118:1340–7.
Kahn SR. The post-thrombotic syndrome. Hematology. 2016;2016:413–8.
Vedantham S. Valvular dysfunction and venous obstruction in the post-thrombotic syndrome. Thromb Res. 2009;123:S62–5.
Bergan JJ, Schmid-Schönbein GW, Smith PDC, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. New Engl J Medicine. 2006;355:488–98.
Passerini AG, Milsted A, Rittgers SE. Shear stress magnitude and directionality modulate growth factor gene expression in preconditioned vascular endothelial cells. J Vasc Surg. 2003;37:182–90.
Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schönbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc. 2004;28:484–93.
Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016;280:97–113.
Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A. The role of endothelial dysfunction and inflammation in chronic venous disease. Ann Vasc Surg. 2018;46:380–93.
Deatrick KB, Elfline M, Baker N, Luke CE, Blackburn S, Stabler C, et al. Postthrombotic vein wall remodeling: Preliminary observations. J Vasc Surg. 2011;53:139–46.
Chandrashekar A, Garry J, Gasparis A, Labropoulos N. Vein wall remodeling in patients with acute deep vein thrombosis and chronic postthrombotic changes. J Thromb Haemost. 2017;15:1989–93.
Rabinovich A, Cohen JM, Kahn SR. The predictive value of markers of fibrinolysis and endothelial dysfunction in the post thrombotic syndrome. Thromb Haemostasis. 2014;111:1031–40.
Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, et al. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arteriosclerosis Thrombosis Vasc Biology. J Am Hear Assoc. 2004;24:1130–7.
Henke PK, Varma MR, Deatrick KB, Dewyer NA, Drewyer NA, Lynch EM, et al. Neutrophils modulate post-thrombotic vein wall remodeling but not thrombus neovascularization. Thromb Haemostasis. 2006;95:272–81.
Baker SK, Strickland S. A critical role for plasminogen in inflammation. J Exp Med. 2020;217: e20191865.
Deatrick KB, Eliason JL, Lynch EM, Moore AJ, Dewyer NA, Varma MR, et al. Vein wall remodeling after deep vein thrombosis involves matrix metalloproteinases and late fibrosis in a mouse model. J Vasc Surg. 2005;42:140–8.
Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arteriosclerosis Thrombosis Vasc Biol. 2008;28:387–91.
Nguyen KP, McGilvray KC, Puttlitz CM, Mukhopadhyay S, Chabasse C, Sarkar R. Matrix Metalloproteinase 9 (MMP-9) regulates vein wall biomechanics in murine thrombus resolution. PLoS ONE. 2015;10: e0139145.
Deatrick KB, Obi A, Luke CE, Elfline MA, Sood V, Upchurch GR, et al. Matrix metalloproteinase-9 deletion is associated with decreased mid-term vein wall fibrosis in experimental stasis DVT. Thromb Res. 2013;132:360–6.
Obi AT, Diaz JA, Ballard-Lipka NL, Roelofs KJ, Farris DM, Lawrence DA, et al. Plasminogen activator-1 overexpression decreases experimental postthrombotic vein wall fibrosis by a non-vitronectin-dependent mechanism. J Thromb Haemost. 2014;12:1353–63.
DeRoo S, Deatrick KB, Henke P. The vessel wall: a forgotten player in post thrombotic syndrome. Thromb Haemostasis. 2010;104:681–92.
Undas A, Natorska J. Improving fibrinolysis in venous thromboembolism: impact of fibrin structure. Expert Rev Hematol. 2019;12:597–607.
Tick LW, Kramer MHH, Rosendaal FR, Faber WR, Doggen CJM. Risk factors for post-thrombotic syndrome in patients with a first deep venous thrombosis. J Thromb Haemost. 2008;6:2075–81.
Ageno W, Piantanida E, Dentali F, Steidl L, Mera V, Squizzato A, et al. Body mass index is associated with the development of the post-thrombotic syndrome. Thromb Haemostasis. 2003;89:305–9.
Roberts LN, Patel RK, Chitongo PB, Bonner L, Arya R. Presenting D-dimer and early symptom severity are independent predictors for post-thrombotic syndrome following a first deep vein thrombosis. Brit J Haematol. 2013;160:817–24.
Roberts LN, Patel RK, Goss DE, Chitongo P, Bonner L, Arya R. Relationship between development of post-thrombotic syndrome and serial ultrasound, D-dimer, and factor VIII activity after a first deep venous thrombosis. J Vasc Surg Venous Lymphatic Disord. 2016;4:28–35.
Dongen CJJ, Prandoni P, Frulla M, Marchiori A, Prins MH, Hutten BA. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost. 2005;3:939–42.
Rabinovich A, Ducruet T, Kahn SR, et al. Development of a clinical prediction model for the postthrombotic syndrome in a prospective cohort of patients with proximal deep vein thrombosis. J Thromb Haemost. 2018;16: 262–70.
Rabinovich A, Cohen JM, Prandoni P, Kahn SR. Association between thrombophilia and the post-thrombotic syndrome: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:14–23.
Prandoni P. Healthcare burden associated with the post-thrombotic syndrome and potential impact of the new oral anticoagulants. Eur J Haematol. 2012;88:185–94.
Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post-thrombotic syndrome. J Vasc Surg. 2013;57:254–61.
Wik HS, Enden TR, Ghanima W, Engeseth M, Kahn SR, Sandset PM. Diagnostic scales for the post-thrombotic syndrome. Thromb Res. 2018;164:110–5.
Milan M, Sarolo L, Callegari E, Vedovetto V, Villalta S, Prandoni P. High rate of inter-observer agreement between professional-rated scores of the Villalta scale for the assessment of the post-thrombotic syndrome. Thromb Res. 2016;144:182–3.
Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost. 2009;7:884–8.
Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies. Circulation. 2014;130:1636–61.
Gautam G, Sebastian T, Klok FA. How to differentiate recurrent deep vein thrombosis from postthrombotic syndrome. Hamostaseologie. 2020;40:280–91.
Müller M, Wolf F, Loewe C, Beitzke D, Zehetmayer S, Gschwandtner ME, et al. Preprocedural imaging modalities in patients undergoing iliocaval venous recanalization and stent placement. Vasc Med. 2023;1:135886.
Helyar VG, Gupta Y, Blakeway L, Charles-Edwards G, Katsanos K, Karunanithy N. Depiction of lower limb venous anatomy in patients undergoing interventional deep venous reconstruction—the role of balanced steady state free precession MRI. Br J Radiology. 2018;91:20170005.
van Dam LF, Dronkers CEA, Gautam G, Eckerbom Å, Ghanima W, Gleditsch J, et al. Magnetic resonance imaging for diagnosis of recurrent ipsilateral deep vein thrombosis. Blood. 2020;135:1377–85.
Makedonov I, Kahn SR, Abdulrehman J, Schulman S, Delluc A, Gross P, et al. Prevention of the postthrombotic syndrome with anticoagulation: a narrative review. Thromb Haemostasis. 2021;1:1.
Bradbury C, Fletcher K, Sun Y, Heneghan C, Gardiner C, Roalfe A, et al. A randomised controlled trial of extended anticoagulation treatment versusstandard treatment for the prevention of recurrent venous thromboembolism (VTE) and post-thrombotic syndrome in patients being treated for a first episode of unprovoked VTE (the ExACT study). Br J Haematol. 2020;188:962–75.
Chitsike RS, Rodger MA, Kovacs MJ, Betancourt MT, Wells PS, Anderson DR, et al. Risk of post-thrombotic syndrome after subtherapeutic warfarin anticoagulation for a first unprovoked deep vein thrombosis: results from the REVERSE study. J Thromb Haemost. 2012;10:2039–44.
Søgaard M, Nielsen PB, Skjøth F, Kjældgaard JN, Coleman CI, Larsen TB. Rivaroxaban versus warfarin and risk of post-thrombotic syndrome among patients with venous thromboembolism. Am J Medicine. 2018;131:787-794.e4.
Hull RD, Pineo GF, Brant R, Liang J, Cook R, Solymoss S, et al. Home therapy of venous thrombosis with long-term LMWH versus usual care: patient satisfaction and post-thrombotic syndrome. Am J Med. 2009;122:762-769.e3.
González-Fajardo JA, Martin-Pedrosa M, Castrodeza J, Tamames S, Vaquero-Puerta C. Effect of the anticoagulant therapy in the incidence of post-thrombotic syndrome and recurrent thromboembolism: comparative study of enoxaparin versus coumarin. J Vasc Surg. 2008;48:953-959.e2.
Daskalopoulos ME, Daskalopoulou SS, Tzortzis E, Sfiridis P, Nikolaou A, Dimitroulis D, et al. Long-term treatment of deep venous thrombosis with a low molecular weight heparin (Tinzaparin): a prospective randomized trial. Eur J Vasc Endovasc. 2005;29:638–50.
Romera A, Cairols MA, Vila-Coll R, Martí X, Colomé E, Bonell A, et al. A RANDOMISED OPEN-LABEL TRIAL COMPARING LONG-TERM SUB-CUTANEOUS LOW-MOLECULAR-WEIGHT HEPARIN COMPARED WITH ORAL-ANTICOAGULANT THERAPY IN THE TREATMENT OF DEEP VENOUS THROMBOSIS. Eur J Vasc Endovasc. 2009;37:349–56.
Poterucha TJ, Libby P, Goldhaber SZ. More than an anticoagulant: do heparins have direct anti-inflammatory effects? Thromb Haemostasis. 2017;117:437–44.
Association EWM. Understanding compression therapy: Position document.
Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. J Vasc Surg. 2012;55:122–8.
Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880–8.
Galanaud J-P, Genty-Vermorel C, Barrellier M-T, Becker F, Jabbour V, Blaise S, et al. 25 mm Hg versus 35 mm Hg elastic compression stockings to prevent post-thrombotic syndrome after deep vein thrombosis (CELEST): a randomised, double-blind, non-inferiority trial. Lancet Haematol. 2022;9:e886–96.
Mol GC, van de Ree MA, Klok FA, Tegelberg MJAM, Sanders FBM, Koppen S, et al. One versus two years of elastic compression stockings for prevention of post-thrombotic syndrome (OCTAVIA study): randomised controlled trial. BMJ. 2016;353: i2691.
ten Cate-Hoek AJ, Amin EE, Bouman AC, Meijer K, Tick LW, Middeldorp S, et al. Individualised versus standard duration of elastic compression therapy for prevention of post-thrombotic syndrome (IDEAL DVT): a multicentre, randomised, single-blind, allocation-concealed, non-inferiority trial. Lancet Haematol. 2018;5:e25-33.
Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693–738.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Elsevier Inc; 2016.
Enden T, Haig Y, Kløw N-E, Slagsvold C-E, Sandvik L, Ghanima W, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–8.
Haig Y, Enden T, Grøtta O, Kløw N-E, Slagsvold C-E, Ghanima W, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3:e64-71.
Notten P, ten Cate-Hoek AJ, Arnoldussen CWKP, Strijkers RHW, de Smet AAEA, Tick LW, et al. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol. 2020;7:e40–9.
Notten P, de Smet AAEA, Tick LW, van de Poel MHW, Wikkeling ORM, Vleming L, et al. CAVA (Ultrasound-Accelerated Catheter-Directed Thrombolysis on Preventing Post-Thrombotic Syndrome) Trial: Long-Term Follow-Up Results. J Am Heart Assoc. 2020;10:e018973.
Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. New Engl J Med. 2017;377:2240–52.
Kearon C, Gu C-S, Julian J, Goldhaber S, Comerota A, Gornik H, et al. Pharmacomechanical catheter-directed thrombolysis in acute femoral-popliteal deep vein thrombosis: analysis from a stratified randomized trial. Thromb Haemostasis. 2019;119:633–44.
Weinberg I, Vedantham S, Salter A, Hadley G, Al-Hammadi N, Kearon C, et al. Relationships between the use of pharmacomechanical catheter-directed thrombolysis, sonographic findings, and clinical outcomes in patients with acute proximal DVT: Results from the ATTRACT Multicenter Randomized Trial. Vasc Med. 2019;24:442–51.
Li W, Kessinger CW, Orii M, Lee H, Wang L, Weinberg I, et al. Time-restricted salutary effects of blood flow restoration on venous thrombosis and vein wall injury in mouse and human subjects. Circulation. 2020;143:1224–38.
Glynn RJ, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. New Engl J Med. 2009;360:1851–61.
Delluc A, Ghanima W, Kovacs MJ, Shivakumar S, Kahn SR, Sandset PM, et al. Prevention of post-thrombotic syndrome with rosuvastatin: a multicenter randomized controlled pilot trial (SAVER). Thromb Res. 2022;213:119–24.
Coccheri S, Mannello F. Development and use of sulodexide in vascular diseases: implications for treatment. Drug Des Dev Ther. 2013;8:49–65.
Palareti G, Barinov V, Urbanek T, Cini M, Li Y-J, Bouslama K, et al. Recurrences and bleeding during extended treatment of patients with venous thromboembolism: results of the international, prospective, observational WHITE study. Int Angiol. 2023.
Andreozzi GM, Bignamini AA, Davì G, Palareti G, Matuška J, Holý M, et al. Sulodexide for the prevention of recurrent venous thromboembolism. Circulation. 2015;132:1891–7.
Luzzi R, Belcaro G, Dugall M, Hu S, Arpaia G, Ledda A, et al. The efficacy of sulodexide in the prevention of postthrombotic syndrome. Clin Appl Thrombosis Hemostasis. 2014;20:594–9.
Martinez‐Zapata MJ, Vernooij RW, Tuma SMU, Stein AT, Moreno RM, Vargas E, et al. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2016;4:003229.
Schastlivtsev I, Lobastov K, Barinov V, Kanzafarova I. Diosmin 600 in adjunction to rivaroxaban reduces the risk of post-thrombotic syndrome after femoropopliteal deep vein thrombosis: results of the RIDILOTT DVT study. Int Angiol. 2020;39:361–71.
Galanaud JP, Abdulrehman J, Lazo-Langner A, Gal GL, Shivakumar S, Schulman S, et al. MUFFIN-PTS trial, Micronized Purified Flavonoid Fraction for the Treatment of Post-Thrombotic Syndrome: protocol of a randomised controlled trial. BMJ Open. 2021;11: e049557.
Rabe E, Partsch H, Hafner J, Lattimer C, Mosti G, Neumann M, et al. Indications for medical compression stockings in venous and lymphatic disorders: an evidence-based consensus statement. Phlebology. 2018;33:163–84.
Ginsberg JS, Hirsh J, Julian J, LaandeVries MV, Magier D, MacKinnon B, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161:2105–9.
Azirar S, Appelen D, Prins MH, Neumann MH, Feiter AN, Kolbach DN. Compression therapy for treating post‐thrombotic syndrome. Cochrane Db Syst Rev. 2019;2019:CD004177.
Kahn SR, Shrier I, Shapiro S, Houweling AH, Hirsch AM, Reid RD, et al. Six-month exercise training program to treat post-thrombotic syndrome: a randomized controlled two-centre trial. Can Med Assoc J. 2011;183:37–44.
Padberg FT, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. J Vasc Surg. 2004;39:79–87.
Sharifi M, Bay RC, Karandish K, Emrani F, Snyder R, D’Silva S, et al. The randomized, controlled ATLANTIS trial of aquatic therapy for chronic venous insufficiency. J Vasc Surg Venous Lymphatic Disord. 2021;9:961–70.
Morling JR, Broderick C, Yeoh SE, Kolbach DN. Rutosides for treatment of post‐thrombotic syndrome. Cochrane Db Syst Rev. 2018;2018:CD005625.
Bignamini AA, Matuška J. Sulodexide for the symptoms and signs of chronic venous disease: a systematic review and meta-analysis. Adv Ther. 2020;37:1013–33.
Carroll BJ, Piazza G, Goldhaber SZ. Sulodexide in venous disease. J Thromb Haemost. 2019;17:31–8.
Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction. Circulation Cardiovasc Interventions. 2018;8:e002772.
Sebastian T, Spirk D, Engelberger R, Dopheide J, Baumann F, Barco S, et al. Incidence of stent thrombosis after endovascular treatment of iliofemoral or caval veins in patients with the postthrombotic syndrome. Thromb Haemostasis. 2019;119:2064–73.
Sista AK, Vedantham S, Kaufman JA, Madoff DC. Endovascular interventions for acute and chronic lower extremity deep venous disease: state of the art. Radiology. 2015;276:31–53.
Masuda EM, Kistner RL, Honolulu. F the D of VS Straub Clinic and Hospital Long-term results of venous valve reconstruction: a four- to twenty-one—year follow-up. J Vasc Surg. 1994;19:391–403.
Ulloa JH, Glickman M. One-year first-in-human success for venovalve in treating patients with severe deep venous insufficiency. Vasc Endovasc Surg. 2022;56:277–83.
Dronkers CEA, Mol GC, Maraziti G, van de Ree MA, Huisman MV, Becattini C, et al. Predicting post-thrombotic syndrome with ultrasonographic follow-up after deep vein thrombosis: a systematic review and meta-analysis. Thromb Haemostasis. 2018;118:1428–38.
Chen C-W, Ting H, Chen P-Y, Weng J-C, Hsu Y-C, Wang S-C, et al. Usefulness of triggered non-contrast-enhanced magnetic resonance angiography in assessing lower extremity venous disease. Medicine. 2021;100:e25809.
Rabinovich A, Cohen JM, Kahn SR. Predictive value of markers of inflammation in the postthrombotic syndrome: a systematic review Inflammatory biomarkers and PTS. Thromb Res. 2015;136:289–97.
Bittar LF, Silva LQ, Orsi FL, Zapponi KCS, Mazetto B, Paula EV, et al. Increased inflammation and endothelial markers in patients with late severe post-thrombotic syndrome. Plos ONE. 2020;15:e0227150.
Borgel D, Bianchini E, Lasne D, Pascreau T, Saller F. Inflammation in deep vein thrombosis: a therapeutic target? Hematology. 2019;24:742–50.
Shbaklo H, Holcroft CA, Kahn SR. Levels of inflammatory markers and the development of the post-thrombotic syndrome. Thromb Haemostasis. 2009;101:505–12.
Wik HS, Jacobsen AF, Mowinckel M-C, Sandset PM. The role of inflammation in post-thrombotic syndrome after pregnancy-related deep vein thrombosis: a population-based, cross-sectional study. Thromb Res. 2016;138:16–21.
Rabinovich A, Cohen JM, Cushman M, Wells PS, Rodger MA, Kovacs MJ, et al. Inflammation markers and their trajectories after deep vein thrombosis in relation to risk of post-thrombotic syndrome. J Thromb Haemost. 2015;13:398–408.
Franciscis S, Gallelli L, Amato B, Butrico L, Rossi A, Buffone G, et al. Plasma MMP and TIMP evaluation in patients with deep venous thrombosis: could they have a predictive role in the development of post-thrombotic syndrome? Int Wound J. 2016;13:1237–45.
Bouman AC, Smits JJM, Cate HT, Cate-Hoek AJT. Markers of coagulation, fibrinolysis and inflammation in relation to post-thrombotic syndrome. J Thromb Haemost. 2012;10:1532–8.
Siudut J, Grela M, Wypasek E, Plens K, Undas A. Reduced plasma fibrin clot permeability and susceptibility to lysis are associated with increased risk of postthrombotic syndrome. J Thromb Haemost. 2016;14:784–93.
Mrozinska S, Cieslik J, Broniatowska E, Undas A. Elevated leptin and decreased adiponectin independently predict the post-thrombotic syndrome in obese and non-obese patients. Sci Rep-uk. 2018;8:6938.
Bouman AC, Cheung YW, Spronk HM, Schalkwijk CG, ten Cate H, ten Wolde M, et al. Biomarkers for post thrombotic syndrome: a case-control study. Thromb Res. 2014;134:369–75.
Bittar LF, Mazetto B, Orsi FLA, Collela MP, Paula EVD, Annichino-Bizzacchi JM. Long-term increased factor VIII levels are associated to interleukin-6 levels but not to post-thrombotic syndrome in patients with deep venous thrombosis. Thromb Res. 2015;135:497–501.
McLeod B, Lim HY, Nandurkar H, Ho P, Wang J. Overall hemostatic potential assay detects risk of progression to post-thrombotic syndrome in anticoagulated patients following deep vein thrombosis. Diagnostics. 2022;12:3165.
Wang J, Lim HY, Brook R, Lai J, Nandurkar H, Ho P. Overall Haemostatic Potential (OHP) assay can risk stratify for venous thromboembolism recurrence in anticoagulated patients. J Thromb Thrombolysis. 2022;55:32–41.
Lee N, Wang J, Brook R, Monagle P, Donnan G, Nandurkar H, et al. The evaluation of overall hemostatic potential assay in patients with COVID-19 infection. Int J Lab Hematol. 2022;44:e219–23.
Wang J, Choy KW, Lim HY, Ho P. Impaired fibrinolytic potential predicts oxygen requirement in COVID-19. J Personalized Medicine. 2022;12:1711.
Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23:3636.
Grimnes G, Horvei LD, Tichelaar V, Brækkan SK, Hansen J-B. Neutrophil to lymphocyte ratio and future risk of venous thromboembolism and mortality: the Tromsø Study. Haematologica. 2016;101:e401–4.
Nery F, Carneiro P, Correia S, Macedo C, Gandara J, Lopes V, et al. Systemic inflammation as a risk factor for portal vein thrombosis in cirrhosis: a prospective longitudinal study. Eur J Gastroenterol Hepatol. 2021;33:e108–13.
Labropoulos N, Gasparis AP, Tassiopoulos AK. Prospective evaluation of the clinical deterioration in post-thrombotic limbs. J Vasc Surg. 2009;50:826–30.
Amin EE, Bistervels IM, Meijer K, Tick LW, Middeldorp S, Mostard G, et al. Reduced incidence of vein occlusion and postthrombotic syndrome after immediate compression for deep vein thrombosis. Blood. 2018;132:2298–304.
Amin E, van Kuijk S, Joore M, Prandoni P, ten Cate H, ten Cate-Hoek A. Development and validation of a practical two-step prediction model and clinical risk score for post-thrombotic syndrome. Thromb Haemostasis. 2018;118:1242–9.
Soares RA, Matielo MF, Neto FCB, Nogueira MP, Almeida RD, Sacilotto R. Comparison of the recanalization rate and postthrombotic syndrome in patients with deep venous thrombosis treated with rivaroxaban or warfarin. Surgery. 2019;166:1076–83.
Prandoni P, Ageno W, Ciammaichella M, Mumoli N, Zanatta N, Imberti D, et al. The risk of post-thrombotic syndrome in patients with proximal deep vein thrombosis treated with the direct oral anticoagulants. Intern Emerg Med. 2020;15:447–52.
Jeraj L, Jezovnik MK, Poredos P. Rivaroxaban versus warfarin in the prevention of post-thrombotic syndrome. Thromb Res. 2017;157:46–8.
Coleman CI, Beyer-Westendorf J, Bunz TJ, Mahan CE, Spyropoulos AC. Postthrombotic Syndrome in Patients Treated With Rivaroxaban or Warfarin for Venous Thromboembolism. Clin Appl Thrombosis Hemostasis. 2018;24:575–82.
Utne KK, Dahm A, Wik HS, Jelsness-Jørgensen LP, Sandset PM, Ghanima W. Rivaroxaban versus warfarin for the prevention of post-thrombotic syndrome. Thromb Res. 2018;163:6–11.
Ferreira T, Huber SC, Martinelli BM, Junior AL, Menezes FH, Orsi FA, et al. Low prevalence of Post-thrombotic syndrome in patients treated with rivaroxaban. Vasc Pharmacol. 2020;124:106608.
Cheung YW, Middeldorp S, Prins MH, Pap AF, Lensing AWA, Cate-Hoek AJT, et al. Post-thrombotic syndrome in patients treated with rivaroxaban or enoxaparin/vitamin K antagonists for acute deep-vein thrombosis. Thromb Haemostasis. 2016;116:733–8.
Yang X, Zhang X, Yin M, Wang R, Lu X, Ye K. Elastic compression stockings to prevent post-thrombotic syndrome in proximal deep venous thrombosis patients without thrombus removal. J Vasc Surg Venous Lymphatic Disord. 2022;10:293–9.
Méan M, Limacher A, Alatri A, Aujesky D, Mazzolai L. Derivation and validation of a prediction model for risk stratification of post-thrombotic syndrome in elderly patients with a first deep vein thrombosis. Thromb Haemostasis. 2018;118:1419–27.
Huang H, Gu J-P, Shi H-F, Shi W-Y, Lu J-Y, Chen L, et al. Assessment of the probability of post-thrombotic syndrome in patients with lower extremity deep venous thrombosis. Sci Rep-uk. 2018;8:12663.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No funding support was received for this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wang, J., Smeath, E., Lim, H.Y. et al. Current challenges in the prevention and management of post-thrombotic syndrome—towards improved prevention. Int J Hematol 118, 547–567 (2023). https://doi.org/10.1007/s12185-023-03651-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03651-6