Abstract
More information is needed regarding the efficacy of SARS-CoV-2 mRNA vaccines in immunocompromised populations, including patients with malignant lymphoma. This study aimed to evaluate humoral responses to the second and third mRNA vaccine doses in 165 lymphoma patients by retrospective analysis of serum SARS-CoV-2 spike protein antibody (S-IgG) titers. Patients with S-IgG titers ≥ 300, 10–300, and ≤ 10 binding antibody units (BAU)/mL were defined as adequate responders, low responders, and non-responders, respectively. S-IgG titers > 10 BAU/mL were considered to indicate seroconversion. After the second dose, 56%, 16%, and 28% of patients were adequate responders, low responders and non-responders, respectively. Multivariate analysis revealed that being an adequate responder after the second dose was associated with receiving the vaccine > 12 months after last chemotherapy, total peripheral lymphocyte count of ≥ 1000/µL, estimated glomerular filtration rate of ≥ 50 mL/min/1.73 m2, and vaccine type (mRNA-1273). After the third dose, patients had significantly higher S-IgG titers and a greater proportion achieved seroconversion. With this third dose, 26% of second-dose non-responders achieved seroconversion and 68% of second-dose low responders became adequate responders. Subsequent SARS-CoV-2 mRNA vaccinations may elicit an immune response in immunocompromised patients who do not initially respond to vaccination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Owing to the successful development of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines with initially high efficacy in preventing SARS-CoV-2 infection [1, 2], it was expected that the coronavirus disease 2019 (COVID-19) pandemic would soon be controlled. However, because of new SARS-CoV-2 variants with increased transmission and immune escape abilities [3], SARS-CoV-2 is far from being eradicated. Although the effectiveness of mRNA vaccines in preventing SARS-CoV-2 infection is reported to be attenuated, severe COVID-19 is still expected to be prevented by vaccination [4]. However, vaccinated patients with hematological malignancies, particularly those receiving chemotherapy, developed serious COVID-19 outcomes compared with vaccinated individuals with an intact immune system [5]. In particular, patients with malignant lymphoma who had received treatment targeting B lymphocytes, such as anti-CD20 antibodies and Bruton’s tyrosine kinase inhibitors (BTKi), showed quite low seroconversion rates [6,7,8,9]. Under such circumstances, booster mRNA vaccination is expected to increase the immune response against SARS-CoV-2 in patients with lymphoma.
This retrospective observational study aimed to evaluate the humoral response to the second (dose 2) and third (dose 3) mRNA vaccine doses by measuring SARS-CoV-2 IgG antibodies using stored samples and its safety profile in patients with lymphoma. In addition, we evaluated the clinical outcomes of patients with COVID-19 in our cohort, along with their acquired humoral responses.
Methods
Patients and methods
This retrospective observational study was conducted at a single institution to evaluate the efficacy and safety of SARS-CoV-2 mRNA vaccines in patients with lymphoma. Patients with lymphoma of any histological subtype, who were under treatment or undergoing regular medical check-ups for their lymphoma at Nagoya City University Hospital (NCUH), and who received at least two doses of the SARS-CoV-2 mRNA vaccine (BNT162b2 or mRNA-1273) were eligible. Other inclusion criteria were as follows: (1) age > 18 years, (2) known vaccine type and time of mRNA vaccination, and (3) available for stored serum sample collection between 7 and 60 days, defined as time point (TP) 1, after dose 2. The timing of sample collection depended on the timing of the patient’s visit to the NCUH for a regular check-up of lymphoma. Patients with a known history of SARS-CoV-2 infection before dose 2 were excluded from this study.
Among eligible patients, serum samples were collected between 91 and 120 days (defined as TP2), 121 and 150 days (TP3), and 151 days or later (TP4) after dose 2. In addition, serum samples between 7 and 90 days (TP5) after dose 3 were collected. The schema of sample collection in this study is shown in Figure S1.
SARS-CoV-2 IgG antibodies against spike (S-IgG) and nucleocapsid (N-IgG) proteins were measured using a highly quantitative and reproducible assay, the HISCL® system (Sysmex Corp., Kobe, Japan), as previously reported [10, 11]. This assay uses a fully automated immunochemistry analyzer based on a chemiluminescence enzyme immunoassay methodology. All SARS-CoV-2 IgG antibodies were measured at an outside laboratory (Sysmex Scientific Laboratories, Kobe, Japan) blinded to the clinical information. Patients with an N-IgG titer ≥ 10 sysmex unit (SU)/mL at TP1 were regarded as having had prior SARS-CoV-2 infection (for more information about the cut-off value of positive N-IgG level, see Supplementary Methods) and were excluded from this study. Furthermore, we also excluded patients if they showed positive N-IgG titers in samples collected after TP2 or later or if the patients were clinically diagnosed with COVID-19 after dose 2. The clinical information of patients was extracted from electronic medical records, up to the cut-off date of July 31, 2022.
Information on patient-reported adverse reactions to doses 2 and 3 was collected using a questionnaire distributed in advance before each mRNA vaccination. Further information regarding patient-reported adverse reactions is provided in the Supplementary Methods.
This study was approved by the Institutional Review Board of the NCUH. Written informed consent was obtained from all participants to use their blood samples. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Analysis
According to the S-IgG titers at TP1 and TP5, patients were classified as non-responders (≤ 10 binding antibody units [BAU]/mL), low responders (> 10 and < 300 BAU/mL), and adequate responders (≥ 300 BAU/mL) to doses 2 and 3 [12]. The cut-off level for defining an adequate responder (≥ 300 BAU/mL) was determined based on the previous reports [13, 14]. Seroconversion was defined as acquiring an S-IgG titer > 10 BAU/mL [12, 14]. S-IgG titers below the sensitivity value (5 BAU/mL) were converted to 1 BAU/mL for statistical analyses. The proportion of non-responders, low responders, and adequate responders after doses 2 and 3 was assessed. In addition, serial changes in the S-IgG titer over time were evaluated using samples obtained at TP1 up to TP4. To compare S-IgG titers at different time points, the geometric mean titer (GMT) obtained at each TP was used. The estimated GMTs of TP2 to TP5 were calculated using mixed-effects models for repeated measures to deal with missing data.
In addition, clinical parameters associated with being an adequate responder to dose 2 were evaluated (for further information on clinical parameters assessed and statistical analysis methodology, see Supplementary Methods).
Patient-reported adverse reactions after mRNA vaccination were aggregated and described, without statistical analysis. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
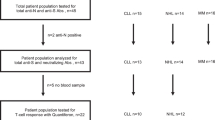
After excluding one patient who had a positive N-IgG titer at TP1, 165 patients with lymphoma were eligible for this study. The patient flowchart is shown in Fig. 1. In Japan, SARS-CoV-2 mRNA vaccination started from April 12, 2021, targeting older adults (≥ 65 years). Patients in our cohort received the first vaccine dose between May 1, 2021 and September 20, 2021.
Patient flowchart showing the progression of patients through the study. TP time point, S-IgG immunoglobulin G antibodies against spike proteins of SARS-CoV-2, N-IgG immunoglobulin G antibodies against nucleocapsid proteins of SARS-CoV-2, TP1 duration defined as within 7–60 days after the second mRNA vaccine dose, TP2 duration defined as within 91–120 days after the second mRNA vaccine dose, TP3 duration defined as within 121–150 days after the second mRNA vaccine dose, TP4 duration defined as within 151 days after the second mRNA vaccine dose until the third vaccine dose, TP5 duration defined as within 7–90 days after the third mRNA vaccine dose, adequate responder, one with an S-IgG titer ≥ 300 binding antibody unit (BAU)/mL; low responder, one with an S-IgG titer of 10–300 BAU/mL; non-responder, one with an S-IgG titer ≤ 10 BAU/mL
The clinical characteristics of the 165 eligible patients are summarized in Table 1. The median age at dose 2 was 71 years (interquartile range [IQR], 63–77). The lymphoma subtypes of the patients were as follows: aggressive B-cell lymphoma (n = 81, 49.1%), indolent B-cell lymphoma (n = 44, 26.7%, including one patient with chronic lymphoid leukemia), peripheral T-cell lymphoma (n = 27, 16.3%, including one patient with mycosis fungoides), and Hodgkin lymphoma (n = 13, 7.9%). Fifty-four patients (32.7%) had received chemotherapy within 12 months before dose 2; 43 of them had received chemotherapy, including anti-CD20 antibodies. Six patients were naïve to chemotherapy before receiving dose 2.
Response to dose 2 of mRNA vaccine
The median duration between dose 2 and TP1 sample collection was 27 days (IQR, 17–39). At dose 2, 148 and 17 patients received BNT162b2 and mRNA-1273, respectively. According to the S-IgG titer measured at TP1, 46 (28%), 26 (16%), and 93 (56%) patients were categorized as non-responders, low responders, and adequate responders, respectively. The median S-IgG titer of 165 patients was 545 BAU/mL (IQR, 1–2237).
Figure 2 shows the effect of the duration between the last anti-CD20 antibody administration and dose 2 on the development of S-IgG titers of each patient. An adequate humoral immune response against SARS-CoV-2 can hardly be expected with dose 2 in patients who received mRNA vaccination within 180 days after the last anti-CD20 antibody treatment; only three (8.6%) achieved seroconversion with the S-IgG titer just slightly above the cut-off value, and no patient became an adequate responder. In contrast, six patients were non-responders, even though they had received the last anti-CD20 antibody > 18 months before dose 2. The clinical characteristics of the patients are presented in Table S1. Notably, four of them were also non-responders to dose 3; the remaining two achieved seroconversion with low titers (20.5 and 27.4 BAU/mL, respectively).
Multivariate logistic regression analysis revealed that becoming an adequate responder was significantly associated with > 12 months of duration between the last chemotherapy administration and dose 2 (odds ratio [OR] 18.14; 95% confidence interval [CI] 6.03–54.59), mRNA-1273 vaccination (OR 8.55; 95% CI 1.33–54.94), absolute lymphocyte count ≥ 1000/µL (OR 3.37; 95% CI 1.14–9.92), and estimated glomerular filtration rate (eGFR) ≥ 50 mL/min/1.73 m2 (OR 4.16; 95% CI 1.31–13.23) (Table 2).
Serial changes in the S-IgG titer over time in patients who achieved seroconversion after dose 2
Serial changes in S-IgG titers of the 119 patients who achieved seroconversion are shown in Figure S2. Among the 119 patients, 50 samples from TP2, 53 samples from TP3, and 64 samples from TP4 were available. The GMT at TP1 was 919 BAU/mL (95% CI 688–1227). The GMT decreased over time until dose 3 was administered. The estimated GMT was 311 BAU/mL (95% CI 228–424) at TP2 (GM ratio to TP1, 0.34; 95% CI 0.28–0.40), 235 BAU/mL (95% CI 171–323) at TP3 (GM ratio to TP1, 0.26; 95% CI 0.21–0.31), and 165 BAU/mL (95% CI 121–225) at TP4 (GM ratio to TP1, 0.18; 95% CI 0.15–0.22).
Response to dose 3 of the mRNA vaccine (booster vaccination)
Serum samples were collected from 116 patients after dose 3 at TP5. The duration between doses 2 and 3 for the 116 patients was 225 days (IQR, 211–236). The median duration of dose 3 and sample collection was 33 days (IQR, 19–52). For dose 3, 89 and 27 patients received BNT162b2 and mRNA-1273, respectively. The other 49 patients were not evaluated for S-IgG titer after dose 3 for the following reasons: five had clinical diagnosis of COVID-19 before dose 3 or were positive for N-IgG, five were lost to follow-up, 17 were without dose 3 administration, three were with unknown status of dose 3, and 19 had received dose 3 but were unavailable for a stored serum sample.
The median S-IgG titer of the 116 patients was 1997 BAU/mL (IQR, 21–6335). The S-IgG titers obtained at TP1 (n = 165) and TP5 (n = 116) are shown in Fig. 3a. According to the S-IgG titer measured at TP5, 26 (22%), 17 (15%), and 73 (63%) patients were categorized as non-responders, low responders, and adequate responders, respectively. The estimated GMT of S-IgG after dose 3 was significantly higher than that obtained after dose 2 (GM ratio to TP1, 3.23; 95% CI 2.35–4.42) (p < 0.001).
a S-IgG titer obtained after the second and third mRNA vaccine doses. b Response to the third mRNA vaccine dose in relation to the response to the second vaccine dose. TP time point, S-IgG immunoglobulin G antibodies against spike proteins of SARS-CoV-2, TP1 duration defined as within 7–60 days after the second mRNA vaccine dose, TP5 duration defined as within 7–90 days after the third mRNA vaccine dose; adequate responder, those with an S-IgG titer ≥ 300 binding antibody unit (BAU)/mL; low responder, one with an S-IgG titer of 10–300 BAU/mL; non-responder, one with an S-IgG titer ≤ 10 BAU/mL; IQR interquartile range, CI confidence interval, GMT geometric mean titer
Of the 34 non-responders to dose 2, nine (26%) achieved seroconversion after dose 3. Of the 19 low responders to dose 2, 13 (68%) became adequate responders (Fig. 3b). In contrast, four adequate responders to dose 2 demonstrated low responses to dose 3; three of them had received rituximab-containing chemotherapy after dose 2, and the remaining one had been receiving lenalidomide maintenance therapy.
Figure S3 shows the effect of the duration between the last anti-CD20 antibody administration and dose 3 on the development of S-IgG titers in each patient. Of the patients who received anti-CD20 antibodies within 180 days before dose 3, three showed S-IgG titers > 10 BAU/mL. However, all were adequate responders to dose 2 and had received dose 2 before the first anti-CD20 antibody administration. Therefore, the S-IgG titer detected after dose 3 was considered to be the remaining S-IgG titer acquired after dose 2 in these three patients.
Patient-reported adverse reactions after dose 2 and 3 of mRNA vaccine
Ninety-nine and 93 patients completed the questionnaire after receiving doses 2 and 3, respectively. The frequencies of all-grade adverse reactions at doses 2 and 3 were very similar; the top three frequent adverse reactions were pain at the injection site (64% and 77%), fatigue (31% and 31%), and fever (28% and 18%) after doses 2 and 3, respectively (Figure S4). The frequency of grade 2/3 adverse reactions was generally low; the most frequent grade 2/3 adverse reaction was fatigue (8.1% and 9.6%, respectively) after doses 2 and 3. Moreover, four of 15 patients and three of 14 patients, who developed any adverse reaction of grade 2/3 after doses 2 and 3, respectively, were non-responders; suggesting that acquisition of humoral response was not associated with stronger adverse reactions to mRNA vaccination.
COVID-19 outcomes in patients with lymphoma who received the mRNA vaccine
Between dose 2 and dose 3, four patients (three adequate responders and one non-responder to dose 2) were clinically diagnosed with COVID-19 between January 2022 and February 2022, when the Omicron epidemic began in Japan. Although the non-responder patient was admitted to the NCUH because of strong fatigue, the severity of COVID-19 was mild, and the patient was treated with sotrovimab and fully recovered. Of the three adequate responders to dose 2, the S-IgG titers obtained at TP3 or TP4 were 192 (obtained 5 days prior to COVID-19 development), 190 (obtained 2 months prior to COVID-19 development), and 1438 BAU/mL (obtained 40 days prior to COVID-19 development), respectively, and all of them recovered without any specific treatment for COVID-19. In addition, another patient (adequate responder to dose 2) was considered to have subclinical SARS-CoV-2 infection, which was revealed by a positive N-IgG titer at TP5. The serum samples after dose 3 in this patient and two of three adequate responders were available; S-IgG titers were prominently elevated ranging 13,200–17,000 BAU/mL with positive N-IgG titer. None of the patients in our cohort died of COVID-19 during the follow-up period.
Discussion
In this study, we comprehensively evaluated humoral responses in patients with lymphoma who had received a second and third dose of the SARS-CoV-2 mRNA vaccine. Furthermore, we evaluated the safety of novel SARS-CoV-2 mRNA vaccines in patients with lymphoma. We found that more patients acquired a higher S-IgG titer after dose 3 of mRNA vaccine than after receiving dose 2. A greater proportion of patients achieved seroconversion after dose 3, which highlights the significance of receiving a booster mRNA vaccination. In contrast, some patients, especially those who had recently received anti-CD20 antibodies, could not acquire an adequate humoral response even after dose 3. We could not evaluate the effect of BTKi on humoral response due to the small number of patients (n = 2) treated with BTKi in our cohort.
In this study, being an adequate responder to dose 2 was associated with parameters such as > 12 months of duration between the last chemotherapy administration and dose 2, total peripheral lymphocyte count ≥ 1000/µL, eGFR ≥ 50 mL/min/1.73 m2, and vaccine type (mRNA-1273). Patients who do not satisfy these parameters at dose 2 may be especially encouraged to receive a booster mRNA vaccination to obtain a higher S-IgG titer, although generally, all patients with lymphoma are encouraged. In the immunocompetent population, several observational studies revealed that mRNA-1273 resulted in higher antibody production [15] in line with our study, and higher effectiveness in preventing SARS-CoV-2 infection than BNT162b2 [16, 17]. However, whether this finding is extrapolated for the third or fourth vaccinations needs to be evaluated. In a large-scale prospective study to evaluate the efficacy and safety of COVID-19 vaccination in patients with kidney disease, patients with chronic kidney disease stages G 4 or 5 (eGFR < 30 mL/min/1.73 m2) demonstrated lower—but not significantly lower—mean S-IgG concentrations compared to controls [13]. On the other hand, among kidney transplant recipients, in a representative immunocompromised population, eGFR levels were significantly lower in non-responders than in responders [13]. The significance of decreased renal function in antibody production against mRNA vaccination in patients with malignant lymphoma needs to be further investigated. We did not assess lymphocyte counts of specific lineages. Previous studies have reported that absolute B-, CD4 T-, or natural killer-cell numbers are associated with humoral responses to mRNA vaccination [7, 12, 18]. Therefore, the higher lymphocyte counts in our patients were possibly associated with the inclusion of those lineages of lymphocytes, which contributed to the acquisition of humoral response. Some studies have reported that lower serum immunoglobulin levels are associated with inadequate humoral responses [7, 19]. However, lower immunoglobulin levels were considered a confounding factor for recent chemotherapy administration and were not identified as significant in our multivariate analysis. We did not evaluate the impact of clinical parameters on the efficacy of dose 3 because it was considered to be largely influenced by the immune status induced by dose 2.
The SARS-CoV-2 mRNA vaccine was initially highly effective in preventing SARS-CoV-2 infection [1, 2] but seems to have limited efficacy in preventing infection against the current Omicron strain of SARS-CoV-2. The vaccine is still expected to prevent severe COVID-19 [4]. In our cohort, four adequate responders to dose 2 were infected with SARS-CoV-2; the COVID-19 severity was mild and resolved without anti-SARS-CoV-2 therapy, although two of them only retained S-IgG titers below the threshold of the adequate titer (300 BAU/mL). This suggests that S-IgG titers alone may not be important in preventing severe COVID-19, in addition to the possibility that SARS-CoV-2 itself has been attenuated by the Omicron strain [4]. As the mRNA vaccine has been reported to induce humoral and cellular immunological memory to the SARS-CoV-2 spike protein [20,21,22,23], it is possible that our patients who previously responded to the mRNA vaccination could develop quick immune responses through immunological memory, which might contribute to the prevention of severe COVID-19. Therefore, although S-IgG titers in our patients dramatically decreased over time, as shown in previous studies including healthy individuals [24], the optimal timing of additional mRNA vaccination may be better determined by considering the memory immunity status.
In contrast, the memory-mediated humoral response is not expected to be triggered by SARS-CoV-2 infection in patients who do not respond to the mRNA vaccine. Such patients are good candidates for receiving the monoclonal-antibody combination (tixagevimab and cilgavimab) for prevention of COVID-19 [25]. In addition, establishing a prompt diagnosis of COVID-19 and timely access to efficient anti-COVID-19 medication [26,27,28] is indispensable.
Furthermore, vaccine-induced T-cell immunity in these patients is expected to prevent severe COVID-19 [18, 21, 29]; nevertheless, further information is needed. Interestingly, some patients in our study experienced strong adverse reactions despite no humoral immunity acquisition. Takano et al. reported that the dynamics of dendritic cell subsets correlated with severity of adverse reactions, not with antibody production by mRNA vaccination [30]. Because dendritic cells play an important role in activating T-cell immune response, our observations were possibly due to the development of cellular immunity via dendritic cell intervention, not just an allergic reaction. Further investigation of the mechanisms of adverse reactions to mRNA vaccines with novel modes of action is warranted.
This study has several limitations. First, because of the small number of patients with COVID-19 in our cohort, the impact of the humoral immunity acquired by the mRNA vaccine on the clinical course of COVID-19 could not be fully assessed. Second, the timing of the measurement of the antibody titers after mRNA vaccination was not uniform; however, it was considered to not affect the determination of the patient being adequate responders. Moreover, we believe that it was ethically unacceptable to increase the number of hospital visits to evaluate antibody production during the COVID-19 pandemic. Finally, we did not evaluate acquired cellular immunity through mRNA vaccination.
Conclusion
This study highlighted the significance of booster SARS-CoV-2 mRNA vaccination in patients with lymphoma and emphasized the importance of establishing effective measures to prevent severe COVID-19 in patients who cannot acquire an adequate humoral response after booster vaccination.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–23.
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16.
Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952–4.
Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T. Clinical severity of, and effectiveness of mRNA vaccines against, Covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376: e069761.
Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader A, et al. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood. 2022;139:1439–51.
Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–73.
Okamoto A, Fujigaki H, Iriyama C, Goto N, Yamamoto H, Mihara K, et al. CD19-positive lymphocyte count is critical for acquisition of anti-SARS-CoV-2 IgG after vaccination in B-cell lymphoma. Blood Adv. 2022;6:3230–3.
Ghione P, Gu JJ, Attwood K, Torka P, Goel S, Sundaram S, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood. 2021;138:811–4.
Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39:1031–3.
Yazaki S, Yoshida T, Kojima Y, Yagishita S, Nakahama H, Okinaka K, et al. Difference in SARS-CoV-2 antibody status between patients with cancer and health care workers during the COVID-19 pandemic in Japan. JAMA Oncol. 2021;7:1141–8.
Noda K, Matsuda K, Yagishita S, Maeda K, Akiyama Y, Terada-Hirashima J, et al. A novel highly quantitative and reproducible assay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies. Sci Rep. 2021;11:5198.
Haggenburg S, Lissenberg-Witte BI, van Binnendijk RS, den Hartog G, Bhoekhan MS, Haverkate NJE, et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022;6:1537–46.
Sanders JF, Bemelman FJ, Messchendorp AL, Baan CC, van Baarle D, van Binnendijk R, et al. The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. 2022;106:821–34.
Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22:1681–91.
Steensels D, Pierlet N, Penders J, Mesotten D, Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–5.
Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27:2136–43.
Dickerman BA, Gerlovin H, Madenci AL, Kurgansky KE, Ferolito BR, Figueroa Muñiz MJ, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in US veterans. N Engl J Med. 2022;386:105–15.
Liebers N, Speer C, Benning L, Bruch PM, Kraemer I, Meissner J, et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients. Blood. 2022;139:142–7.
Narita K, Nakaji S, Tabata R, Terao T, Kuzume A, Tsushima T, et al. Antibody response to COVID-19 vaccination in patients with lymphoma. Int J Hematol. 2022;115:728–36.
Mazzoni A, Di Lauria N, Maggi L, Salvati L, Vanni A, Capone M, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest. 2021;131:e149150
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Barrio DMD, I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–78.
Quast I, Tarlinton D. B cell memory: understanding COVID-19. Immunity. 2021;54:205–10.
Lamacchia G, Mazzoni A, Spinicci M, Vanni A, Salvati L, Peruzzi B, et al. Clinical and immunological features of SARS-CoV-2 breakthrough infections in vaccinated individuals requiring hospitalization. J Clin Immunol. 2022;42:1379–91.
Kato H, Miyakawa K, Ohtake N, Yamaoka Y, Yajima S, Yamazaki E, et al. Vaccine-induced humoral response against SARS-CoV-2 dramatically declined but cellular immunity possibly remained at 6 months post BNT162b2 vaccination. Vaccine. 2022;40:2652–5.
Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–200.
Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–50.
Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386:1397–408.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2022;383:1813–26.
Re D, Seitz-Polski B, Brglez V, Carles M, Graça D, Benzaken S, et al. Humoral and cellular responses after a third dose of SARS-CoV-2 BNT162b2 vaccine in patients with lymphoid malignancies. Nat Commun. 2022;13:864.
Takano T, Morikawa M, Adachi Y, Kabasawa K, Sax N, Moriyama S, et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell Rep Med. 2022;3: 100631.
Acknowledgements
The authors would like to thank C Fukuyama, S Kanie, T Inoue, and all the laboratory department staff of Nagoya City University Hospital for serum sample preparation.
Funding
This study is a collaborative research with Sysmex Corp., Japan. This study was supported in part by the National Cancer Center Research and Development Fund (2020-J-3). Sysmex Corp. was responsible for the cost and implementation of the S-IgG and N-IgG titer evaluation. Sysmex Corp. was involved in the review of the manuscript but not with the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
The conception and design of this study were planned by TS and SK. TS, SK, NN, YN, YK, AK, NM, TE, TN, YM, KO, SK, TN, AI, MR, HK, and SI collected patient information and obtained consent from the patients for this study. TS and HH performed statistical analyses. YK and AT measured S-IgG and N-IgG titers. TS wrote the original draft of this manuscript, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Iida reports research funding and consulting fees from Pfizer. All other authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board of the Nagoya City University Hospital and was conducted in accordance with the principles of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all participants to store their blood samples.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Suzuki, T., Kusumoto, S., Kamezaki, Y. et al. A comprehensive evaluation of humoral immune response to second and third SARS-CoV-2 mRNA vaccination in patients with malignant lymphoma. Int J Hematol 117, 900–909 (2023). https://doi.org/10.1007/s12185-023-03550-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-023-03550-w