Abstract
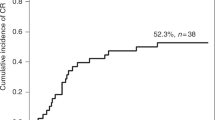
This retrospective study assessed the effectiveness of eltrombopag (EPAG), a thrombopoietin receptor agonist, in the treatment of poor graft function (PGF) following an allogeneic haemopoietic stem cell transplantation (HSCT). Complete response was defined as normalization of blood counts, whereas partial response was defined as transfusion independence. A total of 48 patients with full donor chimerism after HSCT, received EPAG for a median of 120 days (range 10–591). Patients with uni- bi- or tri-lineage cytopenia started treatment at a median of 95 days (range 17–877) after HSCT. The overall response rate was 75%: 24 patients had a complete response and 12 had a partial response. Positive predictors of response were an HLA-matched donor, a CD34+ dose at transplant > 4 × 106/kg, and starting EPAG treatment at least 90 days after HSCT. Patients with more than one positive predictor had a response rate of 92% for the overall patient cohort and 94% for patients with tri-lineage cytopenia. One-year survival was 89% for complete responders, 60% for partial responders and 20% for non-responders (p = 0.0004). EPAG improves peripheral blood counts in patients with poor graft function following HSCT. Response to EPAG can be predicted and has a significant impact on survival.

Similar content being viewed by others
Availability of data and materials
Data are available from the corresponding author on reasonable request.
References
Balassa K, Danby R, Rocha V. Haematopoietic stem cell transplants: principles and indications. Br J Hosp Med. 2019;80(1):33–9.
McSweeney PA, Storb R. Mixed chimerism: preclinical studies and clinical applications. Biol Blood Marrow Transplant. 1999;5(4):192–203.
Murphy KM. Chimerism analysis following hematopoietic stem cell transplantation. In: Czader M, editor. Hematological malignancies. Methods in molecular biology (methods and protocols), vol. 999. Totowa: Humana Press; 2013.
Dominietto A, Raiola AM, van Lint MT, Lamparelli T, Gualandi F, Berisso G, et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol. 2001;112(1):219–27.
Lee KH, Lee JH, Choi SJ, Kim S, Seol M, Lee YS, et al. Failure of trilineage blood cell reconstitution after initial neutrophil engraftment in patients undergoing allogeneic hematopoietic cell transplantation - frequency and outcomes. Bone Marrow Transplant. 2004;33(7):729–34.
Sun Y-Q, He G-L, Chang Y-J, Lan-Ping Xu, Zhang X-H, Han W, et al. The incidence, risk factors, and outcomes of primary poor graft function after unmanipulatedhaploidentical stem cell transplantation. Ann Hematol. 2015;94:1699–705.
Labrador J, López-Corral L, Vazquez L, Sánchez-Guijo F, Guerrero C, Sánchez-Barba M, et al. Incidence and risk factors for life-threatening bleeding after allogeneic stem cell transplant. Br J Haematol. 2015;169(5):719–25.
Guardiola P, Kuentz M, Garban F, Blaise D, Reiffers J, Attal M, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukaemia, chronic myeloid leukaemia and aplastic anaemia. French Society of Bone Marrow Transplantation. Br J Haematol. 2000;111(1):292–302.
Bittencourt H, Rocha V, Filion A, Ionescu I, Herr AL, Garnier F, et al. Granulocyte colony-stimulating factor for poor graft function after allogeneic stem cell transplantation: 3 days of G-CSF identifies long-term responders. Bone Marrow Transplant. 2005;36(5):431–5.
Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20(9):1440–3.
Liu X, Wu M, Peng Y, Chen X, Sun J, Huang F, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014;23(9):1087–98.
Ghobadi A, Fiala MA, Ramsingh G, Gao F, Abboud CN, Stockerl-Goldstein K, et al. Fresh or cryopreserved CD34(+)-selected mobilized peripheral blood stem and progenitor cells for the treatment of poor graft function after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23(7):1072–7.
Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–50.
Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26(3):e65–73.
Rivera D, Bastida JM, Lopez-Corral L, Sanchez-Guijo F, Cabrero M, Martin A, et al. Usefulness of eltrombopag for treating thrombocytopenia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2019;54(5):757–61.
Fu H, Zhang X, Han T, Mo X, Wang Y, Chen H, et al. Eltrombopag is an effective and safe therapy for refractory thrombocytopenia after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54(8):1310–8.
Yuan C, Boyd AM, Nelson J, Patel RD, Varela JC, Goldstein SC, et al. Eltrombopag for treating thrombocytopenia after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25(7):1320–4.
Tang C, Chen F, Kong D, Ma Q, Dai H, Yin J, et al. Successful treatment of secondary poor graft function post allogeneic hematopoietic stem cell transplantation with eltrombopag. J HematolOncol. 2018;11(1):103.
Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, et al. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019;54(8):1346–53.
Raut SS, Shah SA, Sharanangat VV, Shah KM, Patel KA, Anand AS, et al. Safety and efficacy of eltrombopag in post-hematopoietic stem cell transplantation (HSCT) thrombocytopenia. Indian J Hematol Blood Transfus. 2015;31(4):413–5.
Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(5):919–24.
Narita Y, Uchiyama T, Mizumoto C, Takeoka T, Tomo K, Tsuji M, et al. Successful treatment of post-allogeneic hematopoietic transplant immune thrombocytopenia with eltrombopag. Rinsho Ketsueki. 2018;59(11):2418–22.
Master S, Dwary A, Mansour R, Mills GM, Koshy N. Use of eltrombopag in improving poor graft function after allogeneic hematopoietic stem cell transplantation. Case Rep Oncol. 2018;11(1):191–5.
Reid R, Bennett JM, Becker M, Chen Y, Milner L, Phillips GL, et al. Use of eltrombopag, a thrombopoietin receptor agonist, in post-transplantation thrombocytopenia. Am J Hematol. 2012;87(7):743–5.
Fujimi A, Kamihara Y, Hashimoto A, Kanisawa Y, Nakajima C, Hayasaka N, et al. Identification of anti-thrombopoietin receptor antibody in prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation treated successfully with eltrombopag. Int J Hematol. 2015;102(4):471–6.
Dyba J, Tinmouth A, Bredeson C, Matthews J, Allan DS. Eltrombopag after allogeneic haematopoietic cell transplantation in a case of poor graft function and systematic review of the literature. Transfus Med. 2016;26(3):202–7.
Li S, Wu R, Wang B, Fu L, Zhu G, Zhou X, et al. Eltrombopag for delayed platelet recovery and secondary thrombocytopenia following allogeneic stem cell transplantation in children. J PediatrHematolOncol. 2019;41(1):38–41.
Marjanska A, Czyzewski K, Debski R, Krenska A, Wysocki M, Styczynski J. The successful sequential use of plerixafor and eltrombopag for hematopoietic cell transplantation in a child with high-risk neuroblastoma. J PediatrHematolOncol. 2020;42(7):e680–2.
Gao F, Zhou X, Shi J, Luo Yi, Tan Y, Huarui Fu, et al. Eltrombopag treatment promotes platelet recovery and reduces platelet transfusion for patients with post-transplantation thrombocytopenia. Ann Hematol. 2020;99(11):2679–87.
Clark JR, Scott SD, Jack AL, Lee H, Mason J, Carter GI, et al. Monitoring of chimerism following allogeneic haematopoietic stem cell transplantation (HSCT): technical recommendations for the use of short tandem repeat (STR) based techniques, on behalf of the United Kingdom National External Quality Assessment Service for Leucocyte ImmunophenotypingChimerism Working Group. Br J Haematol. 2015;168(1):26–37.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
SG and AB designed the study and read, revised, and approved drafts of the manuscript. SS, PC, LL, FS, MM, AB, AR and CV provided data on patients and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflicts of interest to declare.
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. This retrospective study was approved by the Ethics Committee of Fondazione Policlinico A. Gemelli IRCCS (prot.0030921/20; 23/07/2020).
Informed consent
Every patient gave written informed consent for the use of EPAG as an off-label drug.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Giammarco, S., Sica, S., Chiusolo, P. et al. Eltrombopag for the treatment of poor graft function following allogeneic stem cell transplant: a retrospective multicenter study. Int J Hematol 114, 228–234 (2021). https://doi.org/10.1007/s12185-021-03153-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-021-03153-3