Abstract
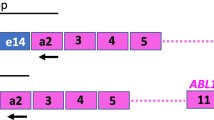
Although monitoring of BCR-ABL1 translocation has become an established practice in the management of chronic myeloid leukemia (CML), the detection limit of the BCR-ABL1 transcripts needs more standardization. The aim of the present study was to evaluate the clinical performances of a novel assay for the quantification of BCR-ABL1 fusion transcripts (e13a2 and e14a2) and ABL1 in a single reaction. This assay is based on the real-time reverse transcription polymerase chain reaction (RT-qPCR) in multiplex format. In a retrospective comparative clinical study performed in a reference laboratory, RNA was extracted from 48 CML patient blood samples with various BCR-ABL1/ABL1 ratios and RT-qPCR was performed using either MAScIR assay or the RT-qPCR simplex reference assay used in routine clinical testing. The comparative clinical results showed high qualitative and quantitative concordance (correlation coefficient >0.95) between MAScIR and the reference assays. The present study illustrates the utility of MAScIR assay as a sensitive, rapid, and cost-effective quantitative device to monitor the BCR-ABL1 ratios by RT-qPCR on whole blood of diagnosed Philadelphia chromosome-positive (Ph+) leukemia patients. This test could be used as an aid in the assessment of molecular response to available treatments.



Similar content being viewed by others
References
Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–3.
Heisterkamp N, Stam K, Groffen J, de Klein A, Grosveld G. Structural organization of the bcr gene and its role in the Philadelphia translocation. Nature. 1985;315:758–61.
Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–82.
Sillaber C, Mayerhofer M, Agis H, Sagaster V, Mannhalter C, Sperr WR, et al. Chronic myeloid leukemia: pathophysiology, diagnostic parameters, and current treatment concepts. Wien Klin Wochenschr. 2003;115:485–504.
Sattler M, Griffin JD. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol. 2003;40:4–10.
Maurer J, Janssen JWG, Thiel E, van Denderen J, Ludwig WD, Aydemir U, et al. Detection of chimeric BCR/ABL genes in acute lymphoblastic leukemia by the polymerase chain reaction. Lancet. 1991;1337:1055–8.
Schlieben S, Borkhardt A, Reinisch I, Ritterbach J, Janssen JW, Ratei R, et al. Incidence and clinical outcome of children with BCR/ABL-positive acute lymphoblastic leukemia (ALL). A prospective RT-PCR study based on 673 patients enrolled in the German pediatric multicenter therapy trials ALL-BFM-90 and CoALL-05-92. Leukemia. 1996;10:957–63.
Buño I, Wyatt WA, Zinsmeister AR, Dietz-Band J, Silver RT, Dewald GW, et al. A special fluorescent in situ hybridization technique to study peripheral blood and assess the effectiveness of interferon therapy in chronic myeloid leukemia. Blood. 1998;92:2315–21.
Schoch C, Schnittger S, Bursh S, Gerstner D, Hochhaus A, Berger U, et al. Comparison of chromosome binding analysis, interphase-and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and follow-up in CML: a study on 350 cases. Leukemia. 2002;16:53–9.
Wang YL, Bagg A, Pear W, Nowell PC, Hess JL. Chronic myeloid leukemia: laboratory diagnosis and monitoring. Genes Chromosomes Cancer. 2001;32:97–111.
Baccarani M, Cortes J, Pane F, Niederwiseser D, Saglio G, Apperley J, et al. Chronic myloide leukemia: an update of concepts and management recommendations of European leukemia Net. J Clin Oncol. 2009;27:6041–51.
Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alpha plus cytarabine in newly diagnosed chronic chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37.
Gaber J, Beillard E, Van Der Velden V, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of of ‘real-time’ quantitative reverse transciptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia. A Europe Against Cancer Program. Leukemia. 2003;17:2318–57.
Muler MC, Saglio G, Lin F, Pfeifer H, Press RD, Tubbs RR, et al. An international study to standardize the detection and quantification of BCR-ABL transcripts from stabilized peripheral blood preparations by quantitative RT-PCR. Haematologica. 2007;92:970–3.
Bradford S, Hughes T. Diagnosis and monitoring of chronic myeloid leukemia by qualitative and quantitative RT-PCR. Methods Mol Med. 2006;125:69–92.
National Comprehensive Cancer network. Clinical practice guideline in oncology: chronic myelogenous leukaemia, version 1. http//nccn.org/professionals/physician_gls/PDF/cml.pdf. Accessed July 2007, following the International Scale proposed previously.
Hughes T, Kaeda J, Branford S, Rudzki Z, Hochhau A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alpha plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32.
Beillard E, Pallisgaard N, Vander Velden V, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate controlgenes for diagnosis and residual disease detection in leukemia patients using ‘real time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR)- a European Against Cancer program. Leukemia. 2003;17:2474–86.
Gabert J, Beillard E, van der Velden VH, Bi W, Grimwade D, Pallisgaard N, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17(12):2318–57.
Moumen A, Dehbi H, Kottwitz H, El Amrani M, H. El Hadi, H. Sefrioui. Comparison of real-time qPCR, conventional PCR and FISH in detecting Mbcr and mbcr translocation in Moroccan chronic myeloid leukaemia patients treated with Imatinib. GMR. 2014. (in press).
White H, Deprez L, Corbisier P, Hall V, Lin F, Mazoua S, et al. A certified plasmid reference material for the standardisation of BCR-ABL1 mRNA quantification by real-time quantitative PCR. Leukemia. 2015;29(2):369–76. doi:10.1038/leu.2014.217.
Branford S, Fletcher L, Cross NC, Müller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112(8):3330–8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Mughal TI, Yong A, Szydio RM, Dazzi F, Olavarria E, van Rhee F, et al. Molecular studies in patients with chronic myeloid leukemia in remission 5 years after allogeneic stem cell transplant define the risk of subsequent relapse. Br J Haematol. 2001;115:569–74.
Press RD, Love Z, Tronnes AA, Trant T, Tran T, Mongoue-Tchokote S, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response (CCR) predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006;107:4250–6.
White HE, Matejtschuck P, Rigsby P, Gabert J, Lin F, Lynn Wang Y, et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantification of BCR-ABL mRNA. Blood. 2010;116:11–117.
Luthra R, Sanchez-Vega B, Medeiros LJ. TaqMan RT-PCR assay coupled with capillary electrophoresis for quantification and identification of bcr-abl transcript type. Mol Pathol. 2004;17:96–103.
Cortes J, Talpaz M, O’Brien S, Jones D, Luthra R, Shan J, et al. Molecular responses in patients with CML phase treated with imatinib mesylate. Clin Cancer. 2005;11:3425–32.
Cervantes F, López-Garrido P, Montero MI, Jonte F, Martínez J, Hernández-Boluda JC, et al. Early intervention during imatinib therapy in patients with newly diagnosed chronic phase chronic myeloid leukemia: a study of the Spanish PETHEMA group. Haematologica. 2010;95(8):1317–24.
CLSI. Evaluation of precision performance of quantitative measurement methods: approved guidelines, 2nd. NCCLS document EP5-A2;2000.
Sefrioui H, El Amrani M and Kottwitz K. Patent WO2014042497A1: www.google.com/patents/WO2014042497A1?cl=en&hl=fr.
Brown JT, Laosinchai-Wolf W, Hedges JB, Watt CD, Van Deerlin VM, Fletcher L, et al. Establishment of a standardized multiplex assay with the analytical performance required for quantitative measurement of BCR–ABL1 on the international reporting scale. Blood Cancer J. 2011;1:e13. doi:10.1038/bcj.2011.10.
Acknowledgments
We are grateful for all the patients who gave their informed consent prior the inclusion of their samples in the study. We also thank all the members of MAScIR medical biotechnology and Pasteur Institute of Morocco for their assistance and help in performing the present work. The study was supported by the Moroccan Foundation for Advanced Sciences and research Innovation and Research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
D. Kottwitz and H. El Hadi authors contributed equally.
About this article
Cite this article
Kottwitz, D., EL Hadi, H., El Amrani, M. et al. Evaluation of a novel multiplex RT-qPCR assay for the quantification of leukemia-associated BCR-ABL1 translocation. Int J Hematol 102, 335–341 (2015). https://doi.org/10.1007/s12185-015-1839-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-015-1839-4