Abstract
Infants (<1 year old) with acute myeloid leukemia (AML) are particularly vulnerable to intensive cytotoxic therapy. Indeed, the mortality rate was high among infants enrolled in the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05 study, which prompted us to temporarily suspend patient enrollment and amend the protocol. Forty-five infants with AML were enrolled. For patients aged <2 years, drug doses were adjusted for body weight. Following the protocol amendments, doses for infants were reduced by a further 33 % in the initial induction course. Six infants died during the induction phase (including five early deaths), mainly due to pulmonary complications. The 3-year probability of overall survival (pOS) in all 45 infants [55.9 %, 95 % confidence interval (CI) 37.9–70.6 %] was significantly lower than that of patients aged 1 to <2 years (77.0 %, 95 % CI 62.7–86.3 %) and those aged ≥2 years (74.7 %, 95 % CI 69.2–79.4 %) (P = 0.037), mainly due to the higher non-relapse mortality rate in infants. No early deaths occurred after the protocol amendments, and the 3-year pOS of the 17 infants enrolled thereafter was 76.4 % (95 % CI 48.8–90.4 %). In conclusion, appropriate dose reduction is essential to avoid early deaths when treating infants with AML.
Similar content being viewed by others
Introduction
Infants aged <1 year with acute myeloid leukemia (AML) show distinct clinical features compared with older children with AML, including a higher white blood cell (WBC) count, extramedullary involvement at diagnosis, and a higher frequency of M4/M5 or M7 leukemic cells classified by the French–American–British (FAB) system, as well as unique cytogenetic characteristics [1, 2]. Relatively few infants have favorable cytogenetic characteristics, such as t(8;21)(q22;q22)/RUNX1-RUNX1T1, inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/CBFB-MYH11, or t(15;17)(q22;q12)/PML-RARA. Most infants have cytogenetic characteristics that are associated with poor prognosis, including rearrangement of the mixed lineage leukemia (MLL) gene on chromosome 11q23, t(1;22)(p13;q13)/RBM15-MKL1, which is highly associated with acute megakaryocytic leukemia in non-Down syndrome infants, and t(7;12)(q36;p13)/HLXB9-ETV6 [3–5]. Hence, infants with AML are usually classified in intermediate (IR) or high-risk (HR) groups, and are treated with an intensive combination chemotherapy regimen based on cytarabine and anthracyclines that is also used for older children. However, as infants are particularly vulnerable to intensive cytotoxic treatment, many study groups have modified the doses of chemotherapeutic drugs administered to infants [6, 7].
In the nationwide multicenter AML-05 study conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG), the early mortality rate was unacceptably high among the first 32 infants (28 eligible infants) enrolled in the study, mainly because of acute respiratory distress syndrome (ARDS). This issue prompted us to temporarily suspend patient enrollment and amend the protocol. Here, we report the outcomes of 45 infants with AML who were enrolled and treated in the AML-05 study.
Materials and methods
Patients
Between November 2006 and December 2010, 485 consecutive patients aged <18 years old with suspected AML treated at 118 institutions in Japan were registered in the AML-05 study. Patients with acute promyelocytic leukemia, Down syndrome, secondary AML, myeloid/natural killer cell leukemia, and myeloid sarcoma were not eligible. AML was diagnosed using the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues (3rd edition) [8] with a comprehensive central diagnostic review system that used morphology, immunophenotyping [9], and cytogenetic analysis of diagnostic bone marrow specimens. Overall, 38 patients (including 5 infants) were excluded, mainly because of misdiagnosis, while 4 additional patients were excluded because the guardian refused to participate (n = 1), there was a significant protocol violation during the initial induction course (n = 1), the hospital withdrew its membership from the JPLSG (n = 1), and the patient was transferred to a non-JPLSG member hospital (n = 1). Among the 443 eligible patients, 45 (10.1 %) were infants aged <1 year at initial diagnosis. Written informed consent, provided according to the Declaration of Helsinki, was obtained from the guardians of each patient. All aspects of the study were approved by the institutional review boards at all participating institutions.
Treatments
The therapeutic regimens used in the AML-05 study are presented in Fig. 1. After the second induction course, the patients were stratified to one of three risk groups according to their cytogenetic characteristics and treatment response following the initial induction course, and received three additional intensified chemotherapeutic courses. Patients who failed to achieve complete remission (CR) after the second course were removed from the study. Allogeneic hematopoietic stem cell transplantation (HSCT) was indicated for all of the high-risk (HR) patients after three or more treatment courses. For patients aged <2 years, the drug doses were reduced by taking into account body weight rather than body surface area throughout the treatment course. Because of the high early mortality rate in infants, we temporarily suspended the enrollment of infants between April 2 and August 11, 2009. At this time, the following amendments were made: (1) an additional dose reduction by 33 % during induction phase 1 for infants; (2) avoidance of the prophylactic administration of granulocyte-colony stimulating factor (G-CSF), considering its possibility as a risk factor for developing ARDS; (3) introduction of enhanced guidelines for supportive care in relation to infection prevention; and (4) close prospective safety monitoring during the induction phase.
Treatment schedule in the AML-05 study. ECM consisted of etoposide (150 mg/m2 per day on days 1–5), cytarabine [200 mg/m2/day via 12 h continuous intravenous (CIV) infusion on days 6–12], mitoxantrone (5 mg/m2/day on days 6–10), and an age-adjusted dose of triple intrathecal chemotherapy (TIT) on day 6. HCEI consisted of high-dose cytarabine (HDCA; 3 g/m2 every 12 h on days 1–3), etoposide (100 mg/m2/day on days 1–5), idarubicin (10 mg/m2 on day 1), and TIT on day 1. HCE consisted of HDCA (2 g/m2 every 12 h on days 1–5), etoposide (100 mg/m2/day on days 1–5), and TIT on day 1. HCI consisted of HCEI without etoposide. HC consisted of HCE without etoposide. HCM consisted of HDCA (2 g/m2 every 12 h on days 1–5), mitoxantrone (5 mg/m2/day on days 1 and 2), and TIT on day 1. Ind-1 induction course 1, Ind-2 induction course 2, Allo HSCT allogeneic hematopoietic stem cell transplantation. Asterisk indicates patients in the intermediate-risk or high-risk groups who experienced Grade 4 infection during intensification course 1 with HCM received HC for intensification course 3
Statistical analyses
The baseline characteristics and the clinical course of patients were analyzed using the χ 2 test or Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for continuous variables. Early death was defined as any-cause death occurring within 42 days of enrollment. Event-free survival (EFS) was defined as the time from the diagnosis of AML to the last follow-up or the first event (failure to achieve remission, relapse, secondary malignancy, or any-cause death). Overall survival (OS) was defined as the time from the diagnosis of AML to any-cause death. The probabilities of EFS (pEFS) and OS (pOS) were estimated using the Kaplan–Meier method. Standard errors (SEs) were calculated using the Greenwood formula and curves were compared using the log-rank test. Confidence intervals (CIs) were calculated at the 95 % confidence level. Gray’s methods were used to estimate and compare the cumulative incidence of important events (relapse, non-relapse death). All analyses were performed using STATA® statistical software (version 11.0; StataCorp LP, College Station, TX). Follow-up data were actualized as of May 1, 2012. This trial is registered with the UMIN Clinical Trials Registry (UMIN-CTR, URL: http://www.umin.ac.jp/ctr/index.htm), number UMIN000000511.
Results
Patient characteristics and clinical outcomes according to age group
The characteristics of the patients are reported in Table 1 for three age groups: infants (<1 year old), 1 to <2 years old, and ≥2 years old. Distributions of FAB categories and cytogenetic characteristics differed among the three groups. In particular, there were more patients with monocytic (M5a/M5b) and megakaryocytic (M7) leukemia, but less with M1/M2 leukemia in the younger age groups. Regarding cytogenetic characteristics, there were more patients with MLL gene rearrangements [t(9;11) and other 11q23 abnormalities], but fewer with core-binding factor AML [t(8;21) and inv(16)/t(16;16)] among infants compared with the other age groups. None of the infants were positive for FLT3 internal tandem duplications.
The results of induction therapies are described in Table 2. The proportion of patients with <5 % bone marrow blasts following initial induction therapy and the complete remission (CR) rate were significantly worse in infants than in patients aged 1 to <2 years or in those aged ≥2 years, respectively. This was due to the higher early mortality rate in infants than in other age groups. CR could not be evaluated in two infants who discontinued the study because of an adverse event (patient #2 in Table 3) and at the physician’s decision.
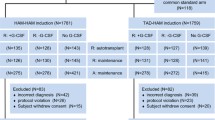
Of the 33 infants who achieved CR, 1 patient with inv(16) was included in the low-risk group, 26 in the IR group, and 5 in the HR group. Four patients in the HR group received allogeneic HSCT at the first CR (Fig. 2). One infant discontinued the study because of the physician’s decision. When we compared the 3-year pEFS among the three age groups, pEFS was lower in infants (46.1 %, 95 % CI 31.1–59.9 %) than in those aged 1 to <2 years (55.4 %, 95 % CI 41.2–67.5 %) or those aged ≥2 years (55.2 %, 95 % CI 49.4–60.5 %). However, the difference was not statistically significant (P = 0.108) because of the relatively small numbers of patients (Fig. 3a). The median follow-up time of living patients was 3.06 years (range, 0.84–5.36 years). However, the 3-year pOS was significantly worse in infants (55.9 %, 95 % CI 37.9–70.6 %) than in those aged 1 to <2 years (77.0 %, 95 % CI 62.7–86.3 %) and those aged ≥2 years (74.7 %, 95 % CI 69.2–79.4 %; P = 0.037; Fig. 3b). The inferior survival rate in infants appeared to be due to the higher non-relapse mortality rate in this age group, rather than recurrent disease (Fig. 3c, d). The non-relapse mortality rates in infants, those aged 1 to <2 years, and those aged ≥2 years were 29.2 % (95 % CI 15.6–50.4 %), 7.4 % (95 % CI 2.8–18.9 %), and 11.8 % (95 % CI 8.3–16.7 %), respectively (P = 0.007). The cumulative relapse rates were 44.0 % (95 % CI 29.8–61.2 %), 41.0 % (95 % CI 29.0–55.8 %), and 42.6 % (95 % CI 37.3–48.3 %), respectively (P = 0.564).
Remission induction results of infants enrolled before and after the protocol amendments
We next compared the outcomes between 28 infants who were enrolled before the protocol amendments and 17 patients enrolled after these amendments. Before the protocol amendments, there were 5 early deaths plus 1 patient who died on day 62 during the initial induction phase (Table 3). These six deaths that occurred during initial induction were due to rapid progression of leukemia in one patient, ARDS in four patients, and interstitial pneumonia in one patient. All five of the non-leukemic deaths were associated with infectious disease or febrile neutropenia. Notably, two of the patients had respiratory syncytial virus (RSV) infection. Concurrent hemophagocytic syndrome (HPS) was also found in three of the patients who died because of non-leukemic causes.
The overall early treatment responses are summarized in Table 2. Although none of the parameters showed statistically significant differences because of the small number of patients analyzed, there were no early deaths after the protocol amendments. Additionally, the incidence of grade ≥3 non-hematological toxicities, as evaluated by the common terminology criteria for adverse events (3rd version), was lower in patients enrolled after versus before the protocol amendments (Table 4). Importantly, it seems that the dose reductions in the initial induction course did not compromise treatment efficacy, as 82.4 % of the infants enrolled after the protocol amendments achieved CR. Additionally, the percentage of patients receiving prophylactic G-CSF use seemed to be lower in infants enrolled after the protocol amendment compared to the pre-amendment cohort; 23.5 % (4/17) vs. 39.2 % (11/28). Median days for G-CSF use in these patients were also shorter in the post-amendment cohort; 9 days (range 5–19 days) vs. 14 days (range 2–25 days).
Overall outcomes of infants enrolled before and after the protocol amendments
There were no significant differences in 3-year pEFS [42.8 % (95 % CI 24.5–59.9 %) vs. 47.0 % (95 % CI 22.9–67.9 %); P = 0.679] and the cumulative relapse rate [41.3 % (95 % CI 23.8–64.8 %) vs. 52.9 % (95 % CI 32.0–77.0 %); P = 0.425] between infants enrolled before and after the protocol amendments (Fig. 4a, c). However, there was a significant improvement in the reduction of non-relapse mortality rate [35.7 % (95 % CI 19.9–58.3 %) vs. 7.1 % (95 % CI 1.0–40.9 %); P = 0.046; Fig. 4d] and achieved 3-year pOS of 76.4 % (95 % CI 48.8–90.4 %) for the post-amendment cohort [vs. 52.7 % (95 % CI 32.8–69.3 %) for the pre-amendment cohort; P = 0.131; Fig 4b]. The incidence of grade ≥3 adverse events by treatment courses in infants is described in Table 4. Besides hematologic toxicities and febrile neutropenia/infection that were frequent throughout the whole treatment courses, the other grade ≥3 adverse events that occurred in >10 % of infants beyond the initial induction course were gastrointestinal toxicity (diarrhea) and elevated liver function parameters (aspartate aminotransferase and alanine aminotransferase).
Discussion
Intensive combination chemotherapy with cytarabine and anthracyclines, together with optimal risk stratification based on the cytogenetic characteristics of leukemia cells and the response to the initial induction course, has led to a 70 % probability of survival in childhood AML [10, 11]. Infants with AML aged <1 year have features that are usually associated with poor prognosis, including high WBC count and/or extramedullary involvement at diagnosis, higher frequencies of monocytic leukemia or megakaryocytic leukemia, and higher frequency of MLL gene rearrangement, and few infants have favorable cytogenetic characteristics, such as t(8;21)(q22;q22)/RUNX1-RUNX1T1 and t(15;17)(q22;q12)/PML-RARA [1–3]. Thus, infants with AML are usually classified in the IR or HR groups and receive treatments appropriate for this level of risk.
Despite these ‘unfavorable’ characteristics, the outcomes of infants with AML are generally not much worse than those of older children with AML. For example, in the Japan Infant Leukemia Study Group, the 3-year pEFS and pOS were 72 and 76 %, respectively, for 35 infants with AML [12]. The AML Berlin–Frankfurt–Münster (BFM) Study Group reported that the 5-year pEFS and pOS were 44 and 61 %, respectively, in the AML-BFM 98 study (n = 59) and 51 and 75 %, respectively, in the AML-BFM 04 study (n = 61)[6]. The 5-year pEFS was 58 % in the United Kingdom (UK) Medical Research Council (MRC) AML-10 and AML-12 studies (n = 151) [7]. In other Japanese AML studies, similar results were reported for infant AML, with 5-year pEFS and pOS of 49.4 and 58.3 % in the Tokyo Children’s Cancer Study Group (TCCSG) M91-13 and M96-14 studies (n = 24) [13] and 46.0 and 64.5 %, respectively, in the Japanese Childhood AML Cooperative Group AML99 study (n = 27) [10]. In the current study, pOS was lower in infants than in the other age groups. However, this disparity was due to the higher non-relapse mortality rate in infants and not the cumulative relapse rate, which was not significantly different among the three age groups.
The high early mortality rate (17.8 %, 5/28) observed in infants enrolled before the protocol amendments was somewhat unexpected, because the induction regimen, which consisted of etoposide (150 mg/m2 per day on days 1–5), cytarabine (200 mg/m2/day via 12 h continuous intravenous infusion on days 6–12), and mitoxantrone (5 mg/m2/day on days 6–10), together with an age-adjusted dose of triple intrathecal chemotherapy on day 6, was identical to the induction regimen used in prior Japanese studies, although the dose modification method differed among these studies [10, 12, 13]. In the TCCSG M91-13 and M96-14 studies, the doses were adjusted for body surface area, with an additional dose reduction of 33 %, and the early mortality rate was 12.5 % (3/24). In the AML99 study, the doses were adjusted for body weight, as in our study before the protocol amendments, and the early mortality rate was 3.7 % (1/27).
It is notable that all non-leukemic deaths during the initial induction course were due to pulmonary complications following infectious complications. Of note, three patients had HPS. These findings suggest that infections in infants are likely to induce hypercytokinemia and result in severe conditions, such as ARDS and/or HPS. Consequently, we also amended the protocol to avoid the prophylactic administration of G-CSF, because this may aggravate inflammatory cytokine production, is often observed in engraftment syndrome after HSCT, and G-CSF was reported to have no benefits in the treatment of children with AML [14–16]. The BFM Study Group reported that, although hemorrhage and leukostasis were the main causes of death within the first 15 days of initial therapy, fatal infections were more common between days 15 and 42. They also reported that acute toxicities during the induction course, particularly severe infection and pulmonary toxicity, were more frequent in infants than in older children [17]. Therefore, the management of infectious complications is vital to prevent early death when treating infants with AML.
To prevent fatal infectious complications, we decided to modify the doses of chemotherapeutic drugs during the initial induction course, by reducing the doses of etoposide, cytarabine, and mitoxantrone by 33 %, but not of intrathecal chemotherapy. However, appropriate dose adjustments for infants are not well documented because very few pharmacokinetic studies have been performed in infants [18, 19]. Many factors may affect drug metabolism in infants, including higher total body water content and extracellular water content, higher unbound active fraction of drugs because of lower affinity of drugs to serum proteins, lower p450 enzyme activity, which could reduce or increase the cytotoxic effects, and lower renal clearance, which could increase systemic exposure of drugs because of reduced tubular and glomerular function until about 6 months of age. In addition, the ratio of body weight to body surface area is lower in infants than in older children. Therefore, if the doses are calculated based on the body surface area, the infants would be exposed to greater amounts of each drug. Thus, prior studies have used arbitrary methods to modify the drug doses for infants, including adjustment for body weight in the BFM [6], CCG-2891 [20], and AML99 [10] studies, while the doses were reduced by 25 % in the MRC AML-10 and AML-12 studies [7].
In addition to reducing the doses of chemotherapeutic drugs, we have also revised the guidelines for general supportive care and infection prevention, by recommending bacterial and fungal prophylaxis, intravenous immunoglobulin therapy to maintain IgG levels ≥500 mg/dl, and the use of rooms with positive air pressure with high-efficacy particulate air (HEPA) filtration. Although the incidence of RSV infection is low among patients with AML, it is associated with high mortality in children with AML [21], and it is well known that infants, both immunocompetent and immunocompromised, are particularly vulnerable to RSV. Although RSV infection may be prevented with palivizumab, a humanized monoclonal antibody that targets the RSV epitope, it was not approved for use in malignant disease in Japan until August 2013, so its use was at the physician’s discretion. An aerosol formulation of the anti-viral agent ribavirin is also effective against RSV infection, but only oral agents have been approved in Japan.
With these aforementioned amendments, most of the grade ≥3 non-hematological adverse events decreased, especially, clinically documented infections and pulmonary complications (Table 4). No more early death was observed and only 7.1 % cumulative incidence of non-relapse mortality was documented among the 17 infants enrolled after the protocol amendments. Although this reduction in early and non-relapse death led to achievement of >70 % pOS for the post-amendment cohort, improvements in pEFS and pOS were not statistically significant. Naturally, there are limitations for the exact explanations due to a relatively small number of patients included in the current study, but one must consider the possibility of increased relapse led by treatment reduction in the post-amendment cohort, although cumulative relapse rate itself was not statistically different.
In conclusion, appropriate dose reduction of chemotherapeutic drugs, particularly in the induction phase, together with enhanced supportive care is essential to prevent non-relapse death when treating AML in infants. As the conventional dose-intensifying approach is difficult to apply for this age group, less toxic agents targeting specific biological features are needed to improve the outcomes of infants with AML.
References
Pui CH, Ribeiro RC, Campana D, Raimondi SC, Hancock ML, Behm FG, et al. Prognostic factors in the acute lymphoid and myeloid leukemias of infants. Leukemia. 1996;10(6):952–6.
Ishii E, Okamura J, Tsuchida M, Kobayashi M, Akiyama Y, Nakahata T, et al. Infant leukemia in Japan: clinical and biological analysis of 48 cases. Med Pediatr Oncol. 1991;19(1):28–32.
Sorensen PH, Chen CS, Smith FO, Arthur DC, Domer PH, Bernstein ID, et al. Molecular rearrangements of the MLL gene are present in most cases of infant acute myeloid leukemia and are strongly correlated with monocytic or myelomonocytic phenotypes. J Clin Investig. 1994;93(1):429–37.
Lion T, Haas OA, Harbott J, Bannier E, Ritterbach J, Jankovic M, et al. The translocation t(1;22)(p13;q13) is a nonrandom marker specifically associated with acute megakaryocytic leukemia in young children. Blood. 1992;79(12):3325–30.
Hauer J, Tosi S, Schuster FR, Harbott J, Kolb HJ, Borkhardt A. Graft versus leukemia effect after haploidentical HSCT in a MLL-negative infant AML with HLXB9/ETV6 rearrangement. Pediatr Blood Cancer. 2008;50(4):921–3.
Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Kremens B, Lehrnbecher T, et al. Favorable outcome in infants with AML after intensive first- and second-line treatment: an AML-BFM study group report. Leukemia. 2012;26(4):654–61.
Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19(12):2130–8.
Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302.
Ohta H, Iwamoto S, Kiyokawa N, Tsurusawa M, Deguchi T, Takase K, et al. Flow cytometric analysis of de novo acute myeloid leukemia in childhood: report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2011;93(1):135–7.
Tsukimoto I, Tawa A, Horibe K, Tabuchi K, Kigasawa H, Tsuchida M, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27(24):4007–13.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–52.
Kawasaki H, Isoyama K, Eguchi M, Hibi S, Kinukawa N, Kosaka Y, et al. Superior outcome of infant acute myeloid leukemia with intensive chemotherapy: results of the Japan Infant Leukemia Study Group. Blood. 2001;98(13):3589–94.
Tomizawa D, Tabuchi K, Kinoshita A, Hanada R, Kigasawa H, Tsukimoto I, et al. Repetitive cycles of high-dose cytarabine are effective for childhood acute myeloid leukemia: long-term outcome of the children with AML treated on two consecutive trials of Tokyo Children’s Cancer Study Group. Pediatr Blood Cancer. 2007;49(2):127–32.
Schmid I, Stachel D, Pagel P, Albert MH. Incidence, predisposing factors, and outcome of engraftment syndrome in pediatric allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2008;14(4):438–44.
Lehrnbecher T, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Creutzig U. Prophylactic human granulocyte colony-stimulating factor after induction therapy in pediatric acute myeloid leukemia. Blood. 2007;109(3):936–43.
Ehlers S, Herbst C, Zimmermann M, Scharn N, Germeshausen M, von Neuhoff N, et al. Granulocyte colony-stimulating factor (G-CSF) treatment of childhood acute myeloid leukemias that overexpress the differentiation-defective G-CSF receptor isoform IV is associated with a higher incidence of relapse. J Clin Oncol. 2010;28(15):2591–7.
Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22(21):4384–93.
Pieters R. Infant acute lymphoblastic leukemia: lessons learned and future directions. Curr Hematol Malig Rep. 2009;4(3):167–74.
Biondi A, Cimino G, Pieters R, Pui CH. Biological and therapeutic aspects of infant leukemia. Blood. 2000;96(1):24–33.
Smith FO, Alonzo TA, Gerbing RB, Woods WG, Arceci RJ. Long-term results of children with acute myeloid leukemia: a report of three consecutive phase III trials by the Children’s Cancer Group: CCG 251, CCG 213 and CCG 2891. Leukemia. 2005;19(12):2054–62.
Sung L, Alonzo TA, Gerbing RB, Aplenc R, Lange BJ, Woods WG, et al. Respiratory syncytial virus infections in children with acute myeloid leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51(6):784–6.
Acknowledgments
This work was supported by a Grant for Clinical Cancer Research and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour, and Welfare of Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tomizawa, D., Tawa, A., Watanabe, T. et al. Appropriate dose reduction in induction therapy is essential for the treatment of infants with acute myeloid leukemia: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol 98, 578–588 (2013). https://doi.org/10.1007/s12185-013-1429-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-013-1429-2