Abstract
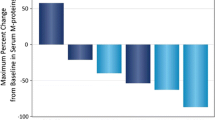
To obtain approval from the Ministry of Health, Labor and Welfare of Japan, a phase II study was conducted to assess the pharmacokinetics and pharmacodynamics of thalidomide along with its efficacy and safety in Japanese patients with multiple myeloma. Between 2005 and 2006, 42 patients were enrolled, and 37 patients met eligibility criteria. Of the 37 patients, 3 were excluded from efficacy analysis because of short duration of thalidomide administration (<4 weeks). The overall response rate was 35.3% (12/34), including partial response of 14.7% (5/34) and minimal response of 20.6% (7/34). The adverse events observed in high frequency (>40%) were leukopenia, neutropenia, drowsiness, dry mouth, and constipation. Grade 3 neutropenia was observed in nine cases. Peripheral neuropathy and eruption were observed in about one-quarter of the patients. Deep vein thrombosis was not observed. At a single oral dose of thalidomide (100 mg), the C max was 1.68 ± 0.41 μg/ml, T max was 4.54 ± 1.71 h, T 1/2 was 4.86 ± 0.44 h, and AUC was 15.87 ± 3.05 μg h/ml. Low-dose thalidomide was an effective and tolerable treatment for Japanese patients with relapsed/refractory myeloma. Leukopenia and neutropenia were the most serious adverse events. The pharmacokinetics was similar to those observed in Caucasian patients.



Similar content being viewed by others
References
Attal M, Harousseau J-L, Facon T, Guilhot F, Doyen C, Fuzibet J-G, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–20. doi:10.1182/blood-2007-10-116129.
Lenz W. Thalidomide embryopathy in Germany, 1959–1961. Prog Clin Biol Res. 1985;163C:77–83.
D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91:4082–5.
Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87:503–8. doi:10.1111/j.1365-2141.1994.tb08304.x.
Singha S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl H Med. 1999;341:1565–71.
Figg WD, Raje S, Bauer KS, Tompkins A, Venzon D, Bergan R, et al. Pharmacokinetics of thalidomide in an elderly prostate cancer population. J Pharm Sci. 1999;88:121–5.
Simmons BR, Lush RM, Figg WD. A reversed-phase high performance liquid chromatography method using solid phase extraction to quantitate thalidomide in human serum. Anal Chim Acta. 1997;339:91–7. doi:10.1016/S0003-2670(96)00494-1.
Barlogie B, Zangari M, Spencer T, Fassas A, Anaissie E, Badros A, et al. Thalidomide in the management of multiple myeloma. Semin Haematol. 2001;38:250–9.
Durie BGM. Low-dose thalidomide in myeloma: efficacy and biologic significance. Semin Oncol. 2002;29 Suppl 17:34–8.
Johnston RE, Abdalla SH. Thalidomide in low doses in effective for the treatment of resistant or relapsed multiple myeloma and for plasma cell leukaemia. Leukemia Lymphoma. 2002;43:351–4.
Murakami H, Handa H, Abe M, Iida S, Ishii A, Ishikawa T, et al. Low-dose thalidomide plus low-dose dexamethasone therapy in patients with refractory multiple myeloma. Eur J Haematol. 2007;79:234–9.
Hattori Y, Okamoto S, Shimada N, Kakimoto T, Morita K, Tanigawara Y, et al. Single-institute phase 2 study of thalidomide treatment for refractory or relapsed multiple myeloma: prognostic factors and unique toxicity profile. Cancer Sci. 2008;99:1243–50.
Matthews SJ, McCoy C. Thalidomide: a review of approved and investigational uses. Clin Therap. 2003;25:356–61.
Rajkumar SV, Rosiñol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–7.
Noormohamed FH, Youle MS, Higgs CJ, Kook KA, Hawkins DA, Lant AF, et al. Pharmacokinetics and hemodynamic effects of single oral doses of thalidomide in asymptomatic human immunodeficiency virus-infected subjects. AIDS Res Human Retroviruses. 1999;15:1047–52.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Murakami, H., Shimizu, K., Sawamura, M. et al. Phase II and pharmacokinetic study of thalidomide in Japanese patients with relapsed/refractory multiple myeloma. Int J Hematol 89, 636–641 (2009). https://doi.org/10.1007/s12185-009-0314-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-009-0314-5