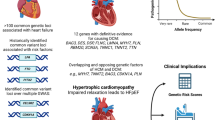
Abstract
Purpose of Review
Over the past 2 decades, our understanding of the role of genetics in the development and progression of aortic disease has grown considerably. This review serves as a foundational text for the reader to gain a thorough understanding of the basics of this emerging field while also displaying the latest breakthroughs which have had, and will continue to have, important impacts on clinical decision-making and patient outcomes. This review focuses on aneurysmal disease, arguably the richest subgroup of aortic diseases for genetic research.
Recent Findings
Thoracic aortic aneurysm and dissection (TAAD) is driven primarily by genetic factors. Genetic variants which drive TAAD tend to occur in genes involved in the structure and function of the aortic wall, specifically the extracellular matrix, TGF-β pathway, and smooth muscle cell contractile units. At least 37 gene variants (pathological or of unknown significance) have been found to be directly associated with the development of TAAD, independent of traditional cardiovascular (CV) risk factors such as smoking and hypertension. The standard suggestion for definitive treatment is prophylactic surgery at an aortic diameter of 5.0–5.5 cm, but several gene variants have been shown to dissect commonly at much smaller diameters (such as TGFβR1/2 or ACTA2), and so mutation-specific guidelines are leading to earlier surgery and better patient outcomes in these aggressive cases.
Abdominal aortic aneurysms (AAAs) also have a genetic component, but these are primarily driven by CV risk factors. Many gene variants have been found which are closely associated with these risk factors, such as those in the lipid metabolism pathway (SORT1). Four variants have been found through GWAS studies to directly influence AAAs independent of general risk factors, though the clinical implications are yet unclear.
Summary
New variants are discovered every year, and each creates a new avenue of research. As more data is collected on patients with each variant, such as the rate of growth, mode of inheritance, age at dissection, and diameter at dissection, we can craft more patient-specific guidelines for different subgroups of affected individuals, with the aim of achieving better long-term survival.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Underlying Cause of Death, 1999–2018 Request. https://wonder.cdc.gov/ucd-icd10.html. Accessed 30 Sep 2020.
Faggion Vinholo, T., Zafar, M. A., Ziganshin, B. A. & Elefteriades, J. A. Nonsyndromic thoracic aortic aneurysms and dissections-is screening possible? Semin Thorac Cardiovasc Surg 31, 628–634 (2019). A detailed analysis of how clinicians can translate knowledge of the prognoses of different mutations into actionable guidelines for screening relatives.
Mariscalco Giovanni, Debiec Radoslaw, Elefteriades John A., Samani Nilesh J., & Murphy Gavin J. Systematic review of studies that have evaluated screening tests in relatives of patients affected by nonsyndromic thoracic aortic disease. Journal of the American Heart Association 7, e009302 (2018). A systematic review looking at the prevalence of specific gene mutations in first, second, and third degree relatives. This has important implications for deciding who to screen.
Svensjö S, et al. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124:1118–23.
Brownstein, A. J., Ziganshin, B. A. & Elefteriades, J. A. Human aortic aneurysm genomic dictionary: is it possible? Indian J Thorac Cardiovasc Surg 35, 57–66 (2019). A discussion on the possibility of creating a genomic “dictionary”- a catalogue of different mutations with different clinical sequelae and guidelines. Features more detail about the characteristics of different mutations.
Ostberg, N. P., Zafar, M. A., Ziganshin, B. A. & Elefteriades, J. A. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 10, (2020).
Biddinger A, Rocklin M, Coselli J, Milewicz DM. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg. 1997;25:506–11.
Coady MA. Familial patterns of thoracic aortic aneurysms. Arch Surg. 1999;134:361.
Faggion Vinholo, T. et al. Genes Associated with thoracic aortic aneurysm and dissection: 2019 update and clinical implications. Aorta (Stamford) 7, 99–107 (2019). An annual update on our group's ongoing work to sequence patients at our center for new/existing variants.
Robertson EN, et al. Familial non-syndromal thoracic aortic aneurysms and dissections — incidence and family screening outcomes. Int J Cardiol. 2016;220:43–51.
Guo D, et al. Genetic variants in LRP1 and ULK4 are associated with acute aortic dissections. Am J Hum Genet. 2016;99:762–9.
Prakash S, LeMaire SA, Bray M, Milewicz DM, Belmont JW. Large deletions and uniparental disomy detected by SNP arrays in adults with thoracic aortic aneurysms and dissections. Am J Med Genet A. 2010;152A:2399–405.
Elefteriades, J. A., Sang, A., Kuzmik, G. & Hornick, M. Guilt by association: paradigm for detecting a silent killer (thoracic aortic aneurysm). Open Heart 2, e000169 (2015).
Luyckx, I., Loeys, B. L., & Curriculum topic: disease of the aorta and trauma to the aorta and heart. The genetic architecture of non-syndromic thoracic aortic aneurysm. Heart 101, 1678–1684 (2015).
Isselbacher, E. M., Lino Cardenas, C. L. & Lindsay, M. E. Hereditary influence in thoracic aortic aneurysm and dissection. Circulation 133, 2516–2528 (2016).
Pomianowski, P. & Elefteriades, J. A. The genetics and genomics of thoracic aortic disease. Annals of Cardiothoracic Surgery 2, 271–279–279 (2013).
Albornoz G, et al. Familial thoracic aortic aneurysms and dissections—incidence, modes of inheritance, and phenotypic patterns. Ann Thorac Surg. 2006;82:1400–5.
Cocciolone AJ, et al. Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2018;315:H189–205.
Davis, E. C. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest 68, 89–99 (1993).
Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Cell biology. Dysfunctional mechanosensing in aneurysms. Science. 2014;344:477–9.
Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907–24.
Karimi A, Milewicz DM. Structure of the elastin-contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Can J Cardiol. 2016;32:26–34.
Andelfinger G, Loeys B, Dietz H. A decade of discovery in the genetic understanding of thoracic aortic disease. Can J Cardiol. 2016;32:13–25.
Pinard, A., Jones, G. T. & Milewicz, D. M. Genetics of thoracic and abdominal aortic diseases: aneurysms, dissections, and ruptures. Circ Res 124, 588–606 (2019). An in-depth look at the pathogenesis several variants by the Milewicz group, including some not covered in this chapter.
Takeda, N. et al. TGF-β signaling-related genes and thoracic aortic aneurysms and dissections. International Journal of Molecular Sciences 19, 2125 (2018). A great resource for studying the precise molecular mechanisms of the TGF-β pathway in the clinical context of thoracic aortic aneurysms.
Jana, S., Hu, M., Shen, M. & Kassiri, Z. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Experimental & Molecular Medicine 51, 1–15 (2019). Contains much more detail about the structure of the aortic wall, along with a review of different ways of modelling it in the context of aortic aneurysms and of the different ECM genes involved in aneurysm formation.
Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241–330.
Rabkin, S. W. Chapter Seven - The role matrix metalloproteinases in the production of aortic aneurysm. in Progress in Molecular Biology and Translational Science (ed. Khalil, R. A.) vol. 147 239–265 (Academic Press, 2017).
Verstraeten A, Alaerts M, Laer LV, Loeys B. Marfan syndrome and related disorders: 25 years of gene discovery. Hum Mutat. 2016;37:524–31.
Dietz HC, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–9.
Judge DP, Dietz HC. Marfan’s syndrome. The Lancet. 2005;366:1965–76.
Regalado ES, et al. Pathogenic FBN1 variants in familial thoracic aortic aneurysms and dissections. Clin Genet. 2016;89:719–23.
Tan L, et al. FBN1 mutations largely contribute to sporadic non-syndromic aortic dissection. Hum Mol Genet. 2017;26:4814–22.
Franken R, et al. Genotype impacts survival in Marfan syndrome. Eur Heart J. 2016;37:3285–90.
Franken Romy et al. Beneficial outcome of losartan therapy depends on type of FBN1 mutation in Marfan syndrome. Circulation: Cardiovascular Genetics 8, 383–388 (2015).
Hiratzka LF, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol. 2010;55:e27–129.
Loeys BL, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–85.
Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–81.
Tran-Fadulu V, et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet. 2009;46:607–13.
Teixidó-Tura G, et al. Heterogeneity of aortic disease severity in patients with Loeys-Dietz syndrome. Heart. 2016;102:626–32.
Jondeau G, et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 Mutations: Results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet. 2016;9:548–58.
Milewicz D, et al. Precision medical and surgical management for thoracic aortic aneurysms and acute aortic dissections based on the causative mutant gene. J Cardiovasc Surg (Torino). 2016;57:172–7.
van de Laar IMBH, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–6.
Schepers, D. et al. A mutation update on the LDS-associated genes TGFB2/3 and SMAD2/3. Hum Mutat 39, 621–634 (2018). This paper contains more details of known mutations in TGFB2/3 and SMAD2/3 which cause Loeys-Dietz Syndrome. It is a great exposition into the way in which basic science integrates well with clinical practice in the field of aortic disease.
Wischmeijer A, et al. Thoracic aortic aneurysm in infancy in aneurysms-osteoarthritis syndrome due to a novel SMAD3 mutation: further delineation of the phenotype. Am J Med Genet A. 2013;161A:1028–35.
Regalado ES, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res. 2011;109:680–6.
van der Linde D, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 Variants. J Am Coll Cardiol. 2012;60:397–403.
Micha D, et al. SMAD2 Mutations are associated with arterial aneurysms and dissections. Hum Mutat. 2015;36:1145–9.
Bertoli-Avella AM, et al. Mutations in a TGF-β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol. 2015;65:1324–36.
Boileau C, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44:916–21.
Guo D-C, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–93.
Milewicz DM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A. 2010;152A:2437–43.
Regalado ES, et al. Aortic disease presentation and outcome associated with ACTA2 mutations. Circ Cardiovasc Genet. 2015;8:457–64.
Bellini C, Wang S, Milewicz DM, Humphrey JD. Myh11(R247C/R247C) mutations increase thoracic aorta vulnerability to intramural damage despite a general biomechanical adaptivity. J Biomech. 2015;48:113–21.
Zhu L, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet. 2006;38:343–9.
Harakalova M, et al. Incomplete segregation of MYH11 variants with thoracic aortic aneurysms and dissections and patent ductus arteriosus. Eur J Hum Genet. 2013;21:487–93.
Wang L, et al. Mutations in myosin light chain kinase cause familial aortic dissections. The American Journal of Human Genetics. 2010;87:701–7.
Gao N, et al. Signaling through myosin light chain kinase in smooth muscles. J Biol Chem. 2013;288:7596–605.
Wallace, S. E. et al. MYLK pathogenic variants aortic disease presentation, pregnancy risk, and characterization of pathogenic missense variants. Genetics in Medicine 21, 144–151 (2019). An in-depth look at MYLK variants and a great example of how new variants should be studied clinically and in the laboratory.
Guo D, et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. The American Journal of Human Genetics. 2013;93:398–404.
Smith LB, et al. Haploinsufficiency of the murine Col3a1 locus causes aortic dissection: a novel model of the vascular type of Ehlers-Danlos syndrome. Cardiovasc Res. 2011;90:182–90.
Shalhub S, et al. Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes. J Vasc Surg. 2014;60:160–9.
Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342:673–80.
Pyeritz, R. E. Ehlers–Danlos Syndrome (2009). https://doi.org/10.1056/NEJM200003093421009https://www.nejm.org/doi/pdf/10.1056/NEJM200003093421009.
Germain DP. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis. 2007;2:32.
Kuang S-Q, et al. FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J Clin Invest. 2016;126:948–61.
Guo D, et al. LOX Mutations predispose to thoracic aortic aneurysms and dissections. Circ Res. 2016;118:928–34.
Lindsay, M. E. & Dietz, H. C. The genetic basis of aortic aneurysm. Cold Spring Harb Perspect Med 4, a015909 (2014).
Kuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015;13:975–87.
Kent, K. C. Clinical practice. Abdominal aortic aneurysms. N Engl J Med 371, 2101–2108 (2014).
Larsson E, et al. High frequency of thoracic aneurysms in patients with abdominal aortic aneurysms. Ann Surg. 2011;253:180–4.
Cheung C, Bernardo AS, Trotter MWB, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin–dependent disease susceptibility. Nat Biotechnol. 2012;30:165–73.
Wahlgren, C. M., Larsson, E., Magnusson, P. K. E., Hultgren, R. & Swedenborg, J. Genetic and environmental contributions to abdominal aortic aneurysm development in a twin population. J Vasc Surg 51, 3–7; discussion 7 (2010).
Johansen K, Koepsell T. Familial tendency for abdominal aortic aneurysms. JAMA. 1986;256:1934–6.
Kuivaniemi H, et al. Familial abdominal aortic aneurysms: collection of 233 multiplex families. J Vasc Surg. 2003;37:340–5.
Jones, G. T. et al. Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ Res 120, 341–353 (2017). The pivotal paper which discovered the new AAA-specific loci which are independent of traditional risk factor genes.
Helgadottir A, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40:217–24.
Kuivaniemi, H., Ryer, E., HR, Y. & Elmore, J. Genetic risk factors for abdominal aortic aneurysms. in 1–30 (2013).
Gretarsdottir S, et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat Genet. 2010;42:692–7.
Harrison SC, et al. Interleukin-6 receptor pathways in abdominal aortic aneurysm. Eur Heart J. 2013;34:3707–16.
Lindeman JHN, et al. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond). 2008;114:687–97.
Interleukin 1 Genetics Consortium. Cardiometabolic effects of genetic upregulation of the interleukin 1 receptor antagonist: a Mendelian randomisation analysis. Lancet Diabetes Endocrinol 3, 243–253 (2015).
Kjolby, M. et al. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab 12, 213–223 (2010).
Zhang Z, Jiang W, Yang H, Lin Q, Qin X. The miR-182/SORT1 axis regulates vascular smooth muscle cell calcification in vitro and in vivo. Exp Cell Res. 2018;362:324–31.
Bradley DT, et al. A variant in LDLR is associated with abdominal aortic aneurysm. Circ Cardiovasc Genet. 2013;6:498–504.
Helgadottir A, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–9.
Galora S, et al. Low-density lipoprotein receptor-related protein 5 gene polymorphisms and genetic susceptibility to abdominal aortic aneurysm. J Vasc Surg. 2013;58:1062-1068.e1.
Du SJ, Tan X, Zhang J. SMYD proteins: key regulators in skeletal and cardiac muscle development and function. Anat Rec (Hoboken). 2014;297:1650–62.
Xu G, et al. The histone methyltransferase Smyd2 is a negative regulator of macrophage activation by suppressing interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) production. J Biol Chem. 2015;290:5414–23.
Fibroblast Growth Factor-9 Activates c-kit progenitor cells and enhances angiogenesis in the infarcted diabetic heart. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4670684/. Accessed 05 Nov 2020.
Matthijs M, et al. Acute elevation of plasma PLTP activity strongly increases pre-existing atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1277–82.
Dryden NH, et al. The transcription factor Erg controls endothelial cell quiescence by repressing activity of nuclear factor (NF)-κB p65. J Biol Chem. 2012;287:12331–42.
Courtois A, et al. 18F-FDG uptake assessed by PET/CT in abdominal aortic aneurysms is associated with cellular and molecular alterations prefacing wall deterioration and rupture. J Nucl Med. 2013;54:1740–7.
Wang, Y. et al. Gene expression signature in peripheral blood detects thoracic aortic aneurysm. PLoS One 2, (2007).
Boileau, A. et al. MiR-574–5p: A circulating marker of thoracic aortic aneurysm. Int J Mol Sci 20, (2019). This paper shows how genetic analysis of aortic aneurysms can help with diagnosis as well as clinical management, in this case by finding circulating miRNAs associated with the disease. As our genetic knowledge grows, so too will the capacity for such innovations to greatly improve clinical care.
Toghill BJ, et al. The potential role of DNA methylation in the pathogenesis of abdominal aortic aneurysm. Atherosclerosis. 2015;241:121–9.
Rice JP, et al. CHRNB3 is more strongly associated with Fagerström test for cigarette dependence-based nicotine dependence than cigarettes per day: phenotype definition changes genome-wide association studies results. Addiction. 2012;107:2019–28.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
John Elefteriades is Principal of CoolSpine, serves on the Data and Safety Monitoring Board of Terumo, and consults for CryoLife. Asanish Kalyanasundaram declares no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Kalyanasundaram, A., Elefteriades, J. The Genetics of Inheritable Aortic Diseases. Curr Cardiovasc Risk Rep 16, 13–24 (2022). https://doi.org/10.1007/s12170-022-00687-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-022-00687-x