Abstract
The change of concentration of various volatiles generated during frying may be an indicator of oil quality. Chemical compounds such as aldehydes, ketones, alcohols and carboxyl acids are the products of oxidation during thermal degradation of edible oils. Shown in this work is a complementary use of GC×GC-TOFMS and PTR-MS for the detection and determination of quality indicators of rapeseed oil. The former technique was used for the identification of potential markers and the latter for their quantitative determination. As a result of the GC×GC analysis, it was determined that 2-pentanone, heptane, octane, 1-heptanol and nonanal can be considered indicators of thermal degradation of rapeseed oil. Using PTR-MS, it was possible to monitor the concentration of these volatile indicators in real time. Based on the results of the analysis and of the reference method, it was concluded that 1-heptanol is best suited for the role of a quality indicator of thermal degradation of rapeseed oil.
Similar content being viewed by others
Introduction
Frying is one of the basic methods of food processing. It contributes to the flavour, aroma, colour and texture of food products (Bordin et al. 2013) and is thus a staple of many cuisines. Frying occurs when the temperature of fat ranges from 150 to 200 °C. The recommended frying temperature for rapeseed oil is 180 °C; however, in practice, it is often exceeded which might lead to the generation of toxic and carcinogenic chemical compounds. The physical properties of fats change significantly during frying. Moreover, numerous chemical reactions such as oxidation, hydrolysis, polymerisation, cyclisation and Maillard’s reactions occur in oil during heating (Choe and Min 2007; Zhang et al. 2012) and products of these might have a detrimental effect on human health. Ingestion of several chemical compounds generated during thermal degradation of edible oils can increase the risk of cancer and of cardiovascular, Alzheimer’s and Parkinson’s diseases (Martínez-Yusta et al. 2014).
In previous studies, it was demonstrated that it is possible to assess the degree of thermal degradation of edible oils based on headspace analysis (Majchrzak et al. 2017; Wojnowski et al. 2017a). During thermal degradation of edible oils, compounds such as carboxylic acids, carbohydrates, ketones, esters, lactones or aldehydes are generated. Some of these compounds, e.g. aldehydes, are volatile products of edible oils’ oxidation (Fullana et al. 2004). For instance, hexanal is recognised as an indicator of the degree of oxidation of rapeseed oil (Matthäus 2006). However, methods such as sensory analysis (IOC 2005), determination of peroxide value (in autooxidation process) (Dieffenbacher and Pocklington 1991) or anisidine value (ISO 6885:1988 1988) remain the standards in vegetable oils’ quality monitoring. In order to determine the product’s thermal stability, the Rancimat method is also commonly used (Mateos et al. 2006). It is important to note that the use of aforementioned techniques does not allow for qualitative or quantitative analysis of the products of the oil’s thermal degradation.
Gas chromatography (GC) is one of the most commonly used instrumental techniques for oil quality assessment. It is utilised in the determination of fatty acids in oils (AOCS 1996) or determination of the headspace composition (García-González et al. 2004). However, at the beginning of the twenty-first century, a new technique, namely multi-dimensional gas chromatography (GC×GC), has emerged. In the recent years, it has been finding application in food analysis (Tranchida et al. 2004; Dymerski et al. 2015). Unlike traditional gas chromatography, the use of GC×GC enables the analysis of relatively complex food matrices due to its superior resolution. Examples of such a complex matrix are edible oils, especially the ones used for thermal treatment. This technique can be used for both targeted and untargeted analysis of vegetable oils (Purcaro et al. 2007) and for the characterisation of fatty acids (de Geus et al. 2001; Mondello et al. 2003). However, there is a lack of examples of the use of multi-dimensional gas chromatography for the assessment of the degree of edible oils’ thermal degradation.
The use of the abovementioned techniques entails certain difficulties. Prior to each analysis, oil has to be sampled and pre-treated which is costly and labour-intensive and constitutes a potential source of measurement errors. Moreover, it is not possible to monitor the changes of the oil’s chemical composition in real time at any given moment of the process of thermal degradation. To this end, proton transfer reaction mass spectrometry (PTR-MS) can be used. This technique was successfully used to classify butter and butter oil (Van Ruth et al. 2007), to determine the geographic origin of olive oil (Araghipour et al. 2008) or to detect oxidative alteration (Aprea et al. 2006).
Presented in this article are the results of research in which a proton transfer reaction mass spectrometer was used to monitor the changes in the concentration of potential indicators of rapeseed oil’s oxidation. The potential indicators were selected based on the results of headspace analysis using two-dimensional gas chromatography-mass spectrometry (GC×GC-MS). To date, the concentration of these compounds was not monitored in real time. The analysis of the dynamic changes in the headspace of oil samples during thermal processing can be an important source of information regarding the processes which occur during frying. Furthermore, using statistical analysis, it was possible to compare the obtained results with induction time determined using the Rancimat method.
Materials and Methods
Materials
The samples of refined rapeseed oil were procured at the local distribution centres in Gdańsk, Poland. They were stored in original, sealed polyethylene packaging at 20 °C in a dark and dry environment to reduce autooxidation. Samples of 5 g of edible oil were placed in 20-mL glass headspace vials and sealed with caps lined with PTFE.
In order to determine potential indicators of the oil’s thermal degradation using GC×GC samples of rapeseed oil were incubated for 24 h at 20, 60, 100, 140 and 180 °C. Samples incubated at different temperatures displayed various degrees of thermal degradation due to the differences in the rate of chemical reactions and thus were of different quality. This experiment was performed in order to maximise the changes occurring during thermal degradation which is crucial for the identification of as many potential volatile markers as possible.
In order to determine the oil’s thermal stability using the Rancimat test and PTR-MS, the samples were placed in a thermostated incubator and heated to 180 °C. The temperature was ramped from 25 to 180 °C over a period of 15 min and then held at 180 °C for an hour. In both procedures, the oil was sampled in 15-min intervals.
Standards used in standard addition calibration method, namely 2-pentanone, heptane, octane, 1-heptanol and nonanal were purchased in Sigma-Aldrich. High-purity water for Rancimat test was obtained using the MilliQ A10 (Millipore).
Rancimat
The determination of edible oil’s oxidative stability using the 893 Professional Biodiesel Rancimat (Metrohm) was used as a reference method. In this method, the measured value is the induction time which can be related to the oil’s quality. During the analysis, the samples were incubated at 110 °C in accordance with ISO 6886:2006. The volumetric flow of air was set to 20 L/h. StabNet software was used to process the results.
HS-SPME GC×GC-TOFMS Analysis
Samples were prepared using headspace solid-phase microextraction (HS-SPME) which is widely used in food analysis for the enrichment of analytes (Wojnowski et al. 2017b). They were incubated for 45 min at 100 °C prior to extraction also for 45 min at 100 °C. High extraction temperature was chosen due to the difficulties with the transfer to the sample’s headspace and subsequent adsorption on the fibre of volatile compounds present in lipophilic matrices such as edible oils. A divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fibre (2 cm, 50/30 μm, Supelco Inc., Bellefonte, USA) was used for the extraction. The use of this type of fibre enables the adsorption of both polar and non-polar chemical compounds. The limitations resulting from a relatively small sorptive area are compensated by the high sensitivity of the GC×GC-TOFMS instrument. Afterwards, the fibre was transferred to the injector of the gas chromatography device where it was thermally desorbed for 4 min at 250 °C.
Comprehensive two-dimensional gas chromatography coupled with mass spectrometry with time-of-flight analyser (GC×GC-TOFMS) was used to analyse the sample. The chromatography setup was comprised of the Agilent 7890A (Agilent Technologies, Santa Clara, USA) equipped with a single-jet dual-stage cryogenic modulator and a split/splitless injector. Pegasus 4D TOFMS (LECO Corp., Saint Joseph, USA) was used as a detector. The 30 m × 0.25 mm × 0.25 μm Equity 1 (non-polar stationary phase—PDMS) (Supelco Inc., Bellefonte, USA) and the 2 m × 0.1 × 0.1 μm SolGel-Wax (polar stationary phase—PEG) (SGE Analytical Science, Ringwood, Australia) chromatographic columns were used. Modulation time was set to 6 s. In the optimised temperature program, the temperature was initially held at 40 °C for 3 min, subsequently ramped to 250 °C at the rate of 5 °C/min and then held for 5 further minutes. The total time of a single analysis was 50 min. The inlet was operated in splitless mode at 250 °C. Hydrogen at a flow rate of 1 mL/min v/v was used as a carrier gas. The temperature of the ion source was set to 250 °C, the detector voltage was 1600 V, and 125 spectra were collected every second. The analysis was run in triplicate for oil samples from three different manufacturers.
PTR-TOFMS
The PTR TOF 1000 ultra (Ionicon GmbH, Innsbruck, Austria) was used for further analysis of oil samples. The samples’ volatile fraction was sampled in a dynamic mode by passing air filtered using the Supelpure® HC Hydrocarbon Trap (Sigma-Aldrich Co.) through the headspace. A nylon syringe filter (0.20 μm, Agilent Technologies, Santa Clara, USA) was placed before the inlet of the PTR-MS. Oil samples were thermostated throughout the analysis. Headspace vials were incubated in a dedicated heating block with a temperature control accuracy of within 0.1 °C. The analytes were introduced into the mass spectrometer through a transfer line heated to 70 °C at 5.0 cm3/min. The detector voltage was set to 2380 V and the remaining parameters were chosen as described in Jordan et al. (2009). Reaction E/N value was set to 130 Td in order to facilitate identification and determination of a wide range of chemical compounds present in the cooking fumes of rapeseed oil. The calculated concentration of selected ions was corrected based on specific reaction rate constants. Averaged mass spectra were recorded every 10 s. IoniTOF v2.4.40 (Ionicon GmbH, Innsbruck, Austria) and PTR-MS Viewer v3.2 (Ionicon GmbH, Innsbruck, Austria) were respectively used for data acquisition and processing.
Data Analysis
Statistical data processing was used in order to reduce the number of variables. The chemometric procedure involved data normalisation, removal of outliers based on the support vector machine algorithm and the selection of 25 most significant chromatographic peaks based on the ReliefF parameter. The ReliefF algorithm is used for binary data classification. It was developed based on the Relief method proposed in 1992 (Kira and Rendell 1992) in which the Manhattan norm is used for finding near-hit and near-miss instances instead of the Euclidean norm. K near misses are selected from each particular class and their contributions are averaged for updating the weight vector (w), based on the prior probability of each class. Data analysis was performed using the Orange v.3.7.0. software (Demšar et al. 2013).
Results
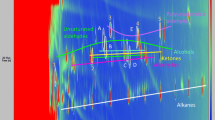
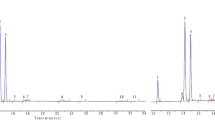
Due to the complex matrix composition and screening character of the measurement, a relatively universal CAR/DVB/PDMS SPME fibre was chosen for the GC×GC analysis. Because of that, both polar and non-polar volatile products of edible oil’s degradation were extracted from the samples. Samples were incubated at five different temperatures for 24 h. Based on the headspace analysis, it was possible to tentatively identify from over 200 volatile chemical compounds in samples incubated at 20 °C to app. 400 volatiles in the headspace of rapeseed oil incubated at 180 °C. Chromatograms (TIC) obtained for samples incubated at 20, 100 and 180 °C are shown in Fig. 1. The compounds present in the samples’ headspace are predominately very volatile and polar substances. Each compound was identified based on selected fragmentation ions listed in the NIST Mass Spectral Library v. 2.0. The next step was to narrow the list of chemical compounds which could be considered as potential indicators of thermal degradation of rapeseed oil.
Compounds the concentration of which had the greatest impact on the classification of samples of rapeseed oil based on the temperature of incubation are listed in Table 1. These 25 chemical compounds fulfil the following criteria:
-
(1)
The signal-to-noise ratio for samples incubated at 180 °C was above 500 for a given unique mass understood as the m/z value of the fragmentation ion the peak area of which was determined. These are the compounds found predominantly in the volatile fraction of the thermally degraded oil.
-
(2)
The changes of concentration of these compounds had the greatest impact on the classification of samples based on the ReliefF algorithm. The ReliefF weight vectors are listed in Table 1.
The number of potential indicators of thermal degradation was further reduced based on the linearity of the changes of their concentration in the samples’ headspace with the increase of incubation temperature.
It was assumed that compounds with corresponding peak area values linearly dependent (with a threshold value of R2 = 0.98) on temperature are possible markers of thermal degradation of rapeseed oil. Using these criteria, five chemical compounds were selected, namely 2-pentanone, heptane, octane, 1-heptanol and nonanal, which are listed in Table 2 together with their retention times in both dimensions, unique mass and correlation of area under the peak with the temperature. The changes of peak area for identified indicators, namely heptane and octane, are depicted in Fig. 2. The peak areas increase with the increase of incubation temperature. Using two-dimensional gas chromatography coupled with TOF detector, it was possible to eliminate the potential coelution which could impact the result of the analysis. Among the identified potential indicators, the highest R2 value was observed for heptane and the lowest for octane.
Furthermore, the concentration of the selected markers was determined in the samples of rapeseed oil using the standard addition calibration (AC) method which allowed compensation for the matrix effect which, in complex gas mixtures such as the analysed samples, can have a considerable impact on the result of the analysis. The concentration of potential indicators was determined in samples incubated at 20, 100 and 180 °C. The results of the analysis are listed in Table 3, together with LOD and LOQ. It should be noted that the concentration of markers in the headspace of samples incubated at 20 °C did not exceed 1 ppm, with the exception of alkanes. This might lead to a conclusion that the rate of oxidation reactions was markedly lower than was the case at higher incubation temperatures. The concentration of markers of the most thermally degraded samples exceeded 5 ppm, with the highest concentration in the samples’ headspace observed for 2-pentanone and the lowest for nonanal.
Using GC×GC-TOFMS, it was possible to obtain low LOD and LOQ values which means that this technique is sufficiently sensitive to determine the products of thermal degradation of edible oils. However, the result of the analysis does not reflect the actual composition of the oil’s headspace during heating or frying, as the concentration of volatiles released during frying is much higher than in the case when the sample is cooled and subsequently heated during solid-phase microextraction. During cooling, the equilibrium between the liquid and gaseous phases changes and the concentration of volatile compounds in the sample’s headspace decreases. Moreover, the SPME process takes place at the same time in three phases: oil, headspace and on the fibre itself.
For that reason, proton transfer reaction mass spectrometry was used which allowed measuring the concentration of VOCs in the oil fumes. However, when using this technique, it is difficult to obtain qualitative information regarding the composition of the sample’s volatile fraction. Since the proton transfer reaction leads to soft ionisation, the obtained m/z signal pertains only to the mass of the pseudo-molecular ion. In the case of compounds with the same or very similar molecular mass, there can be difficulties with their definitive identification and determination. A more reliable qualitative analysis can be performed using hard ionisation, like the one used in GC×GC, as the fragmentation of compounds can reveal information regarding their structure and functional groups. Unfortunately, when using GC×GC for qualitative analysis, it is necessary to perform calibration using standard solutions which is often labour-intensive and might lead to additional measurement errors, and the quantitative analysis is performed indirectly, as opposed to a more direct measurement with PTR-MS, the use of which additionally enables real-time analysis. Thus, both GC×GC-TOFMS and PTR-TOFMS were used as complementary techniques for qualitative and quantitative analysis of the headspace of rapeseed oil samples, enabling comprehensive measurements of the products of thermal degradation in real time.
Using PTR-MS it was possible to monitor the concentration of volatiles generated during thermal degradation of oil. As was the case with GC×GC, it was possible to detect several hundred individual ions in the headspace of rapeseed oil samples. The mass spectra of the volatile fraction of rapeseed oil at the beginning and at the end of the experiment are shown in Fig. 3. It can be observed that the main products of thermal degradation are VOCs with relatively short carbon chains and low molecular weights.
PTR-TOFMS was used for real-time determination of the concentration of previously identified markers of rapeseed oil’s thermal degradation by imitating the processes which occur during frying and which lead to the deterioration of the oil’s quality. Due to the low proton affinity n-alkanes below C6 are not ionised, while higher alkanes, including heptane and octane, react with the H3O+ ion yielding association products [M]H3O+ (Arnold et al. 1998). The remaining determined compounds mostly yield MH+ ions. In order to quantitate the selected compounds, the following k (10−9 cm3/s) values were chosen: 2-pentanone 3.41, heptane 2.17, octane 2.31, 1-heptanol 2.00 and nonanal 3.84. The k values are given according to the Langevin theory, except for nonanal and 2-pentanone which were reported in the literature (Zhao and Zhang 2004). In the absence of literature data, a universal k value of 2.00 was used for 1-heptanol, as it was assumed that the fragmentation of the selected compounds is negligible and the fragmentation ratio remains constant.
The change of the concentration of thermal degradation indicators during the entire analysis is depicted in Fig. 4. In the case of a majority of these compounds, the concentration increases rapidly during heating and then reaches a plateau. It can be assumed that in the case of these volatiles, their presence in the sample’s headspace is caused primarily by diffusion which is more intensive at higher temperatures, and so their concentration in the gaseous phase depends more on the temperature of the process than on its duration. The exception was 1-heptanol, the concentration of which increased throughout the duration of the experiment, making it a possible marker of thermal degradation of rapeseed oil.
The changes of concentration (ppmv, horizontal axis) of selected ions during the incubation of rapeseed oil at 180 °C over a period of 75 min (horizontal axis) measured in real time using PTR-TOFMS. The selected ions correspond to protonated compounds: 2-pentanone, octane, nonanal, heptane and 1-heptanol, respectively
Listed in Table 4 are the concentrations of potential indicators of rapeseed oil’s quality determined during an analysis using PTR-TOFMS. It can be noted that at the beginning of the experiment, the concentration of these compounds did not exceed 1 ppm. Only the concentration of 1-heptanol remained below this threshold throughout the experiment which might present an obstacle in using it as an indicator of thermal degradation, especially when utilising equipment with a lower sensitivity. The results were compared with the induction time measured using the Rancimat test. The induction times at 0, 15, 30, 45, 60 and 75 min of the experiment were 9.62 ± 0.16 h, 6.96 ± 0.53 h, 5.82 ± 0.26 h, 3.97 ± 0.26 h, 0.11 ± 0.05 h and 0.03 ± 0.01 h, respectively. The correlation between induction time and the concentration of possible quality indicators was determined using the Pearson’s test. The best correlation was observed for 1-heptanol, and it can be surmised that its concentration in the rapeseed oil’s volatile fraction is inversely proportional to the induction time which leads to a conclusion that it should be considered an indicator.
Conclusions
In this research, modern instrumental analytical techniques were used to identify the volatile markers of thermal degradation of rapeseed oil. The complementary use of multi-dimensional gas chromatography and proton transfer reaction mass spectrometry is a novel approach in the identification of quality indicators which could be monitored during food processing. In particular, the complementary use of both techniques enables the identification and determination of volatile chemical compounds without the need for the use of calibration standards. This in turn leads to a less labour-intensive approach which, moreover, conforms to the stipulations of Green Analytical Chemistry (Gałuszka et al. 2013). 1-Heptanol, the concentration of which increased from 0.156 ± 0.014 ppmv to 0.933 ± 0.028 ppmv during the course of thermal degradation, was identified as a potential quality indicator of rapeseed oil. The use of PTR-TOFMS enabled real-time monitoring of its concentration and, thanks to the instrument’s high sensitivity, it was possible to identify and determine volatile products of thermal degradation at concentration levels below 1 ppmv, without the need for sample preparation. Moreover, in the described approach, the volatiles are determined not in the sample’s headspace as is the common practice but in the frying fumes in which the concentration of VOCs is much higher and thus the exposure to them is more hazardous. Due to the innovative character of the described approach, the obtained results could not be compared with available subject literature.
The outlined procedure could be used not only for identifying markers of thermal degradation of edible oils but also for selecting volatile indicators from among compounds generated during food processing. In many cases, it is important to monitor the concentration of VOCs in cooking fumes, as they might have a detrimental effect on the health of people who are exposed to them for extended periods of time.
In the future, the described approach will be used for the identification of volatile quality indicators of various edible oils used for frying. The immersion of food products in oil might affect the composition of the volatile fraction and enrich it with products of hydrolysis, or intensify the oxidation due to reactions occurring on the surface of food products.
References
AOCS (1996) Official and tentative methods of the American Oil Chemists’ Society. Method Ca 5a-40, 5th edn. Champaign
Aprea E, Biasioli F, Sani G et al (2006) Proton transfer reaction-mass spectrometry (PTR-MS) headspace analysis for rapid detection of oxidative alteration of olive oil. J Agric Food Chem 54:7635–7640. https://doi.org/10.1021/jf060970r
Araghipour N, Colineau J, Koot A et al (2008) Geographical origin classification of olive oils by PTR-MS. Food Chem 108:374–383. https://doi.org/10.1016/j.foodchem.2007.10.056
Arnold S, Viggiano A, Morris R (1998) Rate constants and product branching fractions for the reactions of H3O+ and NO+ with C-2-C-12 alkanes. J Phys Chem A 102:8881–8887. https://doi.org/10.1021/jp9815457
Bordin K, Kunitake MT, Aracava KK, Trindade CSF (2013) Changes in food caused by deep fat frying—a review. Arch Latinoam Nutr 63:5–13
Choe E, Min DB (2007) Chemistry of deep-fat frying oils. J Food Sci 72:1–10
de Geus H-J, Aidos I, de Boer J et al (2001) Characterisation of fatty acids in biological oil samples using comprehensive multidimensional gas chromatography. J Chromatogr A 910:95–103
Demšar J, Curk T, Erjavec A et al (2013) Orange: data mining toolbox in python. J Mach Learn Res 14:2349–2353
Dieffenbacher A, Pocklington WD (1991) Standard methods for the analysis of oils, fats and derivatives. Int Union Pure Appl Chem 1991:1–151. https://doi.org/10.1351/pac198254061257
Dymerski T, Namieśnik J, Vearasilp K et al (2015) Comprehensive two-dimensional gas chromatography and three-dimensional fluorometry for detection of volatile and bioactive substances in some berries. Talanta 134:460–467. https://doi.org/10.1016/J.TALANTA.2014.11.061
Fullana A, Carbonell-Barrachina ÁA, Sidhu S (2004) Volatile aldehyde emissions from heated cooking oils. J Sci Food Agric 84:2015–2021. https://doi.org/10.1002/jsfa.1904
Gałuszka A, Migaszewski Z, Namieśnik J (2013) The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal Chem 50:78–84. https://doi.org/10.1016/j.trac.2013.04.010
García-González DL, Barie N, Rapp M, Aparicio R (2004) Electronic nose based on metal oxide semiconductor sensors as a fast alternative for the detection of adulteration of virgin olive oils. https://doi.org/10.1021/JF048833M
IOC (2005) Method for the organoleptic assessment of extra virgin olive oil applying to use a designation of origin. 1–29
ISO 6885:1988 (1988) Animal and vegetable fats and oils—determination of anisidine value
Jordan A, Haidacher S, Hanel G et al (2009) A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int J Mass Spectrom 286:122–128. https://doi.org/10.1016/j.ijms.2009.07.005
Kira K, Rendell L (1992) The feature selection problem: traditional methods and a new algorithm. In: AAAI’92 proceedings of the tenth national conference on artificial intelligence. AAAI Press. San Jose, California, pp 129–134
Majchrzak T, Lubinska M, Różańska A et al (2017) Thermal degradation assessment of canola and olive oil using ultra-fast gas chromatography coupled with chemometrics. Monatshefte fur Chemie 148:1625–1630. https://doi.org/10.1007/s00706-017-1968-y
Martínez-Yusta A, Goicoechea E, Guillén MD (2014) Aldehydes after prolonged heating at frying temperature. Elsevier Inc.
Mateos R, Uceda M, Aguilera MP et al (2006) Relationship of Rancimat method values at varying temperatures for virgin olive oils. Eur Food Res Technol 223:246–252. https://doi.org/10.1007/s00217-005-0185-9
Matthäus B (2006) Utilization of high-oleic rapeseed oil for deep-fat frying of French fries compared to other commonly used edible oils. Eur J Lipid Sci Technol 108:200–211. https://doi.org/10.1002/ejlt.200500249
Mondello L, Casilli A, Tranchida PQ et al (2003) Detailed analysis and group-type separation of natural fats and oils using comprehensive two-dimensional gas chromatography. J Chromatogr A 1019:187–196. https://doi.org/10.1016/S0021-9673(03)01130-0
Purcaro G, Morrison P, Moret S et al (2007) Determination of polycyclic aromatic hydrocarbons in vegetable oils using solid-phase microextraction-comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. J Chromatogr A 1161:284–291. https://doi.org/10.1016/j.chroma.2007.05.105
Tranchida PQ, Dugo P, Dugo G, Mondello L (2004) Comprehensive two-dimensional chromatography in food analysis. J Chromatogr A 1054:3–16. https://doi.org/10.1016/j.chroma.2004.07.095
Van Ruth SM, Koot A, Akkermans AW et al (2007) Butter and butter oil classification by PTR-MS. Eur Food Res Technol 227:307–317. https://doi.org/10.1007/s00217-007-0724-7
Wojnowski W, Majchrzak T, Dymerski T et al (2017a) Portable electronic nose based on electrochemical sensors for food quality assessment. Sensors 17:2715. https://doi.org/10.3390/s17122715
Wojnowski W, Majchrzak T, Dymerski T et al (2017b) Dynamic headspace sampling as an initial step for sample preparation in chromatographic analysis. J AOAC Int 100:1559–1606. https://doi.org/10.5740/jaoacint.17-0206
Zhang Q, Saleh ASM, Chen J, Shen Q (2012) Chemical alterations taken place during deep-fat frying based on certain reaction products: a review. Chem Phys Lipids 165:662–681
Zhao J, Zhang R (2004) Proton transfer reaction rate constants between hydronium ion (H3O +) and volatile organic compounds. Atmos Environ 38:2177–2185. https://doi.org/10.1016/j.atmosenv.2004.01.019
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tomasz Majchrzak declares that he has no conflict of interest. Wojciech Wojnowski declares he has no conflict of interest. Tomasz Dymerski declares that he has no conflict of interest. Jacek Gębicki declares that he has no conflict of interest. Jacek Namieśnik declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Majchrzak, T., Wojnowski, W., Dymerski, T. et al. Complementary Use of Multi-dimensional Gas Chromatography and Proton Transfer Reaction Mass Spectrometry for Identification of Rapeseed Oil Quality Indicators. Food Anal. Methods 11, 3417–3424 (2018). https://doi.org/10.1007/s12161-018-1318-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1318-7