Abstract
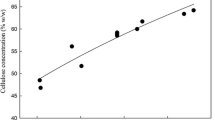
Due to the high potential of the extrusion technique for pretreatment of lignocellulosic substrates, several attempts have been conducted in previous studies to further increase the subsequent sugar yield from extrusion pretreatment. Examples include application of chemicals along with extrusion, such as alkali-extrusion and ethylene glycol-extrusion, or before extrusion, such as hot water extraction. In this study, a new sequential technique has been developed for pretreatment of corn stover (CS), which utilizes an initial extrusion pretreatment (155 rpm screw speed and temperatures of 90°C, 180°C and 180°C corresponding to feed, barrel and die zones, respectively at a reaction time of 45–90 s) followed by pretreatment with polyethylene glycol 6,000 (PEG). In order to fully characterize the response for sugar yield over the range of surfactant treatment conditions assessed, response surface methodology was used. Treatment temperature, incubation time and PEG concentration were varied between 45–55°C, 1–4 h, 0.15–0.6 g PEG/g glucan, respectively. Statistical analysis was conducted by fitting the glucose and xylose yields to a quadratic polynomial model. PEG concentration and temperature were found to be the most significant factors in surfactant pretreatment. The optimum condition resulted in 25.4% and 10.3% increase in glucose and xylose yield, respectively. Using the combination of 10.8 FPU/g glucan of Ctec2 and 0.3 g PEG/g glucan, the glucose yield of extruded CS reached 98%. A yield was 64% resulted from application of similar amounts of Ctec and Htec. Decreased adsorption of enzyme to the lignocellulosic substrate as well as increased enzyme activity and reaction velocity indicated by kinetic parameter evaluation and nitrogen combustion analysis suggested an increased solubilization of cellulase in the presence of PEG. We propose that a non-productive adsorption of enzymes occur during hydrolysis not only due to lignin but also due to crystalline cellulose. Comparison of enzyme adsorptions and increase in sugar yields between Avicel and CS suggests the presence of other potential mechanisms of action for PEG in addition to increase of enzyme solubilization.




Similar content being viewed by others
References
Galbe M, Zacchi G (2002) A review of the production of ethanol from softwood. Appl Microb Biotechnol 59:618–628
Wyman CE (1999) Biomass ethanol; technical progress, opportunities, and commercial challenges. Annu Rev Energy Environ 24:1899–226
Ferreira SMP, Duarte AP, Queiroz JA, Domingues FC (2009) Influence of buffer systems on Trichoderma ressei Rut C-30 morphology and cellulase production. Electronic Journal of Biotechnol 12:1–9
Ma AZ, Hu Q, Qu YB, Bai ZH, Liu WF, Zhuang GQ (2008) The enzymatic hydrolysis rate of cellulose decrease with irreversible adsorption of cellobiohydrolase I. Enzym and Microb Technol 42:543–547
Holtzapple M, Cognata M, Shu Y, Hendrickson C (1990) Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnol Bioeng 36:275–287
Xiao ZZ, Zhang X, Gregg DJ, Saddler JN (2004) Effects of sugar inhibition on cellulases and beta-glucosidase during enzymatic hydrolysis of softwood substrates. Appl Biochem Biotechnol 113–16:1115–1126
Qing Q, Yang B, Wyman CE (2010) Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresource Technol 101:9624–9630
Kim TH, Lee YY, Sunwoo C, Kim JS (2006) Pretreatment of corn stover by low liquid ammonia recycle percolation process. Appl Biochem Biotechnol 133:41–57
Zhu YM, Kim TH, Lee YY, Chen RG, Elander RT (2006) Enzymatic production of xylooligosaccharides from corn stover and corn cobs treated with aqueous ammonia. Applied Biochem and Biotechnol 130:586–598
Ximenes E, Kim Y, Mosier N, Dien B, Ladisch M (2011) Deactivation of cellulases by phenols. Enzyme Microb Technol 48:54–60
Aymard C, Belarbi A (2000) Kinetics of thermal deactivation of enzymes: a simple three parameters phenomenological model can describe the decay of enzyme activity, irrespectively of the mechanism. Enzyme Microb Technol 27:612–618
Kim MH, Lee SB, Ryu DDY (1982) Surface deactivation of cellulose and its prevention. Enzyme Microb Technol 4:99–103
Castanon M, Wilke CR (1981) Effect of the surfactant Tween 80 on enzymatic hydrolysis of newspaper. Biotechnol Bioeng 23:1365–1372
Parke JW, Takahata Y, Kajuchi T, Akehata T (1992) Effects of non-ionic surfactant on enzymatic hydrolysis of used newspaper. Biotechnol Bioeng 39:117–120
Yang SWZ, Quian JQY, Zhou YG, Xia XH (2009) Simple approach for efficient encapsulation of enzyme in silica matrix with retained bioactivity. Analytical Chem 7:3478–3484
Pavani m, Basil DK (2010) Immobilization of Cellulase and Hemicellulases on Porous Glass Beads. ASTM 7: 10 pages.
Yang B, Wyman CE (2006) BSA treatment to enhance enzymatic hydrolysis of cellulose in lignin containing substrate. Wiley Interscience 94:611–617
Zheng Y, Pan Z, Zhang R (2008) Non-ionic surfactants and non-catalytic protein treatment on enzymatic hydrolysis of pretreated creeping wild ryegrass. Appl Biochem Biotechnol 146:231–248
Borjesson J, Engqvist M, Slipos B, Tjerneld F (2007) Effect of polyethylene glycol on enzymatic hydrolysis and adsorption of cellulose enzymes to pretreated lignocellulose. Enzyme Microb Technol 41:186–195
Steele BR, Nghiem SJ, Stowers M (2005) Enzyme recovery and recycling following hydrolysis of ammonia fiber explosion-treated corn stover. Appl Biochem Biotechnol 901:121–124
Qi B, Chen X, Su Y, Wan Y (2011) Enzyme adsorption and recycling during hydrolysis of wheat straw lignocellulose. Bioresource Technol 102:2881–2889
Taherzadeh MJ, Karimi K (2007) Enzyme based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources 4:707–738
Erriksson T, Karlsson J, Tjerland F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 3:353–364
Kumar R, Wyman CE (2008) Effect of additive on the digestibility of corn stover solids following pretreatment by leading technologies. Biotechnol Bioeng 102:1544–1557
Kaar WK, Holtzapple MT (1998) Benefits from Tween during enzymatic hydrolysis of corn stover. Biotechnol & Bieng 59:419–427
Badley RA, Carruthers L, Phillips MC (1977) Hydrophobic free energy and the denaturation of proteins. Biochem Biophys Acta 495:110–113
Kim S, Holtzapple MT (2006) Effect of structural features on enzyme digestibility of corn stover. Bioresource Technol 97:583–591
Lee SH, Inoue S, Teramato Yand Endo T (2010) Enzymatic saccharification of woody biomass micro/nano fibrillated by continues extrusion process II. Effect of hot-compressed water treatment. Bioresource Technol 101:9645–9649
Lee SH, Inoue S, Teramato Yand Endo T (2009) Enzymatic saccharification of woody biomass micro/nanofibrillated by continoius process I—effect of additives with cellulose affinity. Bioresource Technol 100:275–279
Karunanithy C, Muthukumarappan K (2011) Optimization of alkali soaking and extrusion pretreatment of prairie cordgrass for maximum sugar recovery by enzymatic hydrolysis. Biochem Eng J 54:71–82
Karunanithy C, Muthukumarappan K (2011) Optimization of alkali, big blue stem particle size and extruder parameters for maximum enzymatic sugar recovery using response surface methodology. Bioresources 6:762–790
Karunanithy C, Muthukumarappan K (2011) Optimization of alkali, switchgrass, and extruder parameters for maximum sugar recovery. Chemical Eng Technol 34:1413–1426
Eckard AD, Muthukumarappan K, Gibbons WR (2011) Optimization of Tween 20 and SDS pretreatment condition for enzymatic hydrolysis of extruded corn. ASABE Paper no. 1110677. ASABE, Louisville.
Karunanithy C, Muthukumarappan K (2011) Optimization of corn stover and extruder parameters for enzymatic hydrolysis using response surface methodology. Biol Eng 3 (2) Accepted.
Kaya F, Heitmann JA, Joyce TW (1995) Influence of surfactants on the enzymatic hydrolysis of xylan and cellulose. Tappi J 78:149–157
Qi B, Chen X, Wan Y (2010) Pretreatment of wheat straw by non-ionic surfactant assisted dilute acid for enhancing enzymatic hydrolysis and ethanol production. Bioresource Technol 101:4875–4883
Box GEP, Hunter JS (1957) Multi-factor experimental-designs for exploring response surfaces. Ann Math Stat 28:195–241
Obeng DP, Morrell S, Napier-Munn TJ (2005) Application of central composite rotatable design to modelling the effect of some operating variables on the performance of the three-product cyclone. Int J Miner Process 76:181–192
Pedersen M, Johansen SK, Meyerl AS (2011) Low temperature lignocellulose pretreatment: effects and interactions of pretreatment pH are critical for maximizing enzymatic monosaccharide yields from wheat straw. Biotechnol for biofuels 4:1–11
Penner MH (2001) Expression and measurement of enzyme activity. Current protocols in food analytical chem C1.1.1–C1.1.4.
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Liu H, Zhu JY (2010) Eliminating inhibition of enzymatic hydrolysis by lignosulfonate in unwashed sulfite-pretreated aspen using metal salts. Bioresource Technol 101:9120–9127
Karlstrom G (1985) A new model for upper and lower critical solution temperature in poly ethylene oxide solutions. J Phys Chem 89:4962–4964
Saeki SN, Kuwahara M, Nakata MK (1976) Upper and lower critical solution temperature in poly ethylene glycol solutions. Polymer 17:685–189
Huyan JK, Sung BK, Chang JK (2007) The effect of nonionic surfactants on the pretreatment and enzymatic hydrolysis of recycled newspaper. Biotechnol Bioprocess Eng 12:147–151
Chauve M, Maaths H, Delphine H, Casanave D, Monot F, Ferreira NL (2010) Comparative kinetic analysis of two fungal β-glucosidase. Biotechnol Biofuels 3:1–8
Holtzapple MT, Caram SH, Humphrey AE (1984) A comparison of two empirical models for the enzymic hydrolysis of pretreated poplar wood. Biotechnol Bioeng 26:936–941
Cristenson JB, Borejsson J, Bruun MH, Tjerneld F, Jorgensen H (2006) Use of surfactant active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme and Microbial Technol 40:888–895
Acknowledgments
Funding support was provided by a Sun Grant project titled “Development of pretreatment strategies”. The authors would also like to thank Novozymes Inc. for providing the commercial enzymes used in this study. We would also thank Dr. Karunanithy who helped in the extrusion of corn stover.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eckard, A.D., Muthukumarappan, K. & Gibbons, W. Pretreatment of Extruded Corn Stover with Polyethylene Glycol to Enhance Enzymatic Hydrolysis: Optimization, Kinetics, and Mechanism of Action. Bioenerg. Res. 5, 424–438 (2012). https://doi.org/10.1007/s12155-011-9162-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-011-9162-2