Abstract
Objective
The aim of this study is to assess tumor differentiation using parameters from sequential positron emission tomography/computed tomography (PET/CT) and magnetic resonance imaging (MRI) in patients with breast cancer.
Methods
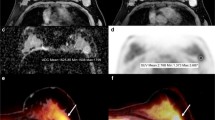
This retrospective study included 78 patients with breast cancer. All patients underwent sequential PET/CT and MRI. For fluorodeoxyglucose (FDG)-PET image analysis, the maximum standardized uptake value (SUVmax) of FDG was assessed at both 1 and 2 h and metabolic tumor volume (MTV) and total lesion glycolysis (TLG). The kinetic analysis of dynamic contrast-enhanced MRI parameters was performed using dynamic enhancement curves. We assessed diffusion-weighted imaging (DWI)–MRI parameters regarding apparent diffusion coefficient (ADC) values. Histologic grades 1 and 2 were classified as low-grade, and grade 3 as high-grade tumor.
Results
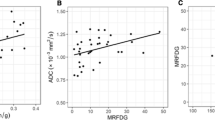
Forty-five lesions of 78 patients were classified as histologic grade 3, while 26 and 7 lesions were grade 2 and grade 1, respectively. Patients with high-grade tumors showed significantly lower ADC-mean values than patients with low-grade tumors (0.99 ± 0.19 vs.1.12 ± 0.32, p = 0.007). With respect to SUVmax1, MTV2.5, and TLG2.5, patients with high-grade tumors showed higher values than patients with low-grade tumors: SUVmax1 (7.92 ± 4.5 vs.6.19 ± 3.05, p = 0.099), MTV2.5 (7.90 ± 9.32 vs.4.38 ± 5.10, p = 0.095), and TLG2.5 (40.83 ± 59.17 vs.19.66 ± 26.08, p = 0.082). However, other parameters did not reveal significant differences between low-grade and high-grade malignancies. In receiver-operating characteristic (ROC) curve analysis, ADC-mean values showed the highest area under the curve of 0.681 (95%CI 0.566–0.782) for assessing high-grade malignancy.
Conclusions
Lower ADC-mean values may predict the poor differentiation of breast cancer among diverse PET–MRI functional parameters.




Similar content being viewed by others
References
Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45(1):1–14.
Ahn SH, Korean Breast Cancer S. Clinical characteristics of breast cancer patients in Korea in 2000. Arch Surg. 2004;139(1):27–30. discussion 1.
Rakha EA, El-Sayed ME, Lee AH, Elston CW, Grainge MJ, Hodi Z, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26(19):3153–8.
Pereira H, Pinder SE, Sibbering DM, Galea MH, Elston CW, Blamey RW, et al. Pathological prognostic factors in breast cancer. IV: Should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology. 1995;27(3):219–26.
Dunnwald LK, Doot RK, Specht JM, Gralow JR, Ellis GK, Livingston RB, et al. PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res. 2011;17(8):2400–9.
Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266(2):388–405.
Sanli Y, Kuyumcu S, Ozkan ZG, Isik G, Karanlik H, Guzelbey B, et al. Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med. 2012;26(4):345–50.
Gil-Rendo A, Martinez-Regueira F, Zornoza G, Garcia-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96(2):166–70.
Garcia Vicente AM, Amo-Salas M, Relea Calatayud F, Munoz Sanchez Mdel M, Pena Pardo FJ, Jimenez Londono GA, et al. Prognostic role of early and end-of-neoadjuvant treatment 18F-FDG PET/CT in patients with breast cancer. Clin Nucl Med. 2016;41(7):e313-22.
Kim J, Yoo SW, Kang SR, Cho SG, Oh JR, Chong A, et al. Prognostic significance of metabolic tumor volume measured by (18)F-FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46(4):278–85.
Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E. Dual time point FDG-PET/CT imaging… Potential tool for diagnosis of breast cancer. Clin Radiol. 2008;63(11):1213–27.
Mavi A, Urhan M, Yu JQ, Zhuang H, Houseni M, Cermik TF, et al. Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med. 2006;47(9):1440–6.
Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246(1):116–24.
Li L, Wang K, Sun X, Wang K, Sun Y, Zhang G, et al. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit. 2015;21:376–82.
Leong LC, Gombos EC, Jagadeesan J, Fook-Chong SM. MRI kinetics with volumetric analysis in correlation with hormonal receptor subtypes and histologic grade of invasive breast cancers. AJR Am J Roentgenol. 2015;204(3):W348-56.
Koo HR, Cho N, Song IC, Kim H, Chang JM, Yi A, et al. Correlation of perfusion parameters on dynamic contrast-enhanced MRI with prognostic factors and subtypes of breast cancers. J Magn Reson Imaging. 2012;36(1):145–51.
Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55(5):736–42.
Brix G, Kiessling F, Lucht R, Darai S, Wasser K, Delorme S, et al. Microcirculation and microvasculature in breast tumors: pharmacokinetic analysis of dynamic MR image series. Magn Reson Med. 2004;52(2):420–9.
Lim I, Noh WC, Park J, Park JA, Kim HA, Kim EK, et al. The combination of FDG PET and dynamic contrast-enhanced MRI improves the prediction of disease-free survival in patients with advanced breast cancer after the first cycle of neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41(10):1852–60.
Byun BH, Noh WC, Lim I, Lee SS, Cho AR, Park JA, et al. A new method for apparent diffusion coefficient measurement using sequential (18)F-FDG PET and MRI: correlation with histological grade of invasive ductal carcinoma of the breast. Ann Nucl Med. 2013;27(8):720–8.
Lim I, Noh WC, Kim H-A, Park KW, Lee SS, Kim KM, et al. Use of dynamic contrast-enhanced MR parameters selected from PET image to predict response to neoadjuvant chemotherapy in advanced breast cancer; parallel PET/MRI. J Nucl Med. 2015;56(supplement 3):569-.
Pickles MD, Manton DJ, Lowry M, Turnbull LW. Prognostic value of pre-treatment DCE-MRI parameters in predicting disease free and overall survival for breast cancer patients undergoing neoadjuvant chemotherapy. Eur J Radiol. 2009;71(3):498–505.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10.
Cipolla V, Santucci D, Guerrieri D, Drudi FM, Meggiorini ML, de Felice C. Correlation between 3T apparent diffusion coefficient values and grading of invasive breast carcinoma. Eur J Radiol. 2014;83(12):2144–50.
Belli P, Costantini M, Bufi E, Giardina GG, Rinaldi P, Franceschini G, et al. Diffusion magnetic resonance imaging in breast cancer characterisation: correlations between the apparent diffusion coefficient and major prognostic factors. Radiol Med. 2015;120(3):268–76.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espie M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38(3):426–35.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38(4):250–8.
Radjenovic A, Dall BJ, Ridgway JP, Smith MA. Measurement of pharmacokinetic parameters in histologically graded invasive breast tumours using dynamic contrast-enhanced MRI. Br J Radiol. 2008;81(962):120–8.
Satake H, Nishio A, Ikeda M, Ishigaki S, Shimamoto K, Hirano M, et al. Predictive value for malignancy of suspicious breast masses of BI-RADS categories 4 and 5 using ultrasound elastography and MR diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196(1):202–9.
Kitajima K, Yamano T, Fukushima K, Miyoshi Y, Hirota S, Kawanaka Y, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol. 2016;85(5):943–9.
Yabuuchi H, Matsuo Y, Okafuji T, Kamitani T, Soeda H, Setoguchi T, et al. Enhanced mass on contrast-enhanced breast MR imaging: Lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. J Magn Reson Imaging. 2008;28(5):1157–65.
Wangerin KA, Muzi M, Peterson LM, Linden HM, Novakova A, O’Sullivan F, et al. Effect of 18F-FDG uptake time on lesion detectability in PET imaging of early stage breast cancer. Tomography. 2015;1(1):53–60.
Tan SL, Rahmat K, Rozalli FI, Mohd-Shah MN, Aziz YF, Yip CH, et al. Differentiation between benign and malignant breast lesions using quantitative diffusion-weighted sequence on 3 T MRI. Clin Radiol. 2014;69(1):63–71.
Qu RF, Guo DR, Chang ZX, Meng J, Sun Y, Hao SH, et al. Differential diagnosis of benign and malignant breast tumors using apparent diffusion coefficient value measured through diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2015;39(4):513 – 22.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, J.H., Lim, I., Noh, W.C. et al. Prediction of tumor differentiation using sequential PET/CT and MRI in patients with breast cancer. Ann Nucl Med 32, 389–397 (2018). https://doi.org/10.1007/s12149-018-1259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-018-1259-7