Abstract
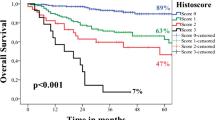
The Risk Model is a validated outcome predictor for patients with head and neck squamous cell carcinoma (Brandwein-Gensler et al. in Am J Surg Pathol 20:167–178, 2005; Am J Surg Pathol 34:676–688, 2010). This model may potentially shift treatment paradigms for patients with low-stage cancers, as current protocols dictate that they might receive only primary surgery. Here we test the hypothesis that the Risk Model has added prognostic value for low-stage oral cavity squamous cell carcinoma (OCSCC) patients. 299 patients with Stage I/II OCSCC were characterized according to the Risk Model (Brandwein-Gensler et al. in Am J Surg Pathol 20:167–178, 2005; Am J Surg Pathol 34:676–688, 2010). Cumulative incidence and competing risk analysis were performed for locoregional recurrence (LRR) and disease-specific survival (DSS). Receiver operating characteristic analyses were performed for worst pattern of invasion (WPOI) and the risk categories. 292 patients were analyzed; 30 T1N0 patients (17 %) and 26 T2N0 patients (23 %) developed LRR. Disease-specific mortality occurred in 9 T1N0 patients (6 %) and 9 T2N0 patients (10 %). On multivariable analysis, the Risk Model was significantly predictive of LRR (p = 0.0012, HR 2.41, 95 % CI 1.42, 4.11) and DSS (p = 0.0005, HR 9.16, 95 % CI 2.65, 31.66) adjusted for potential confounders. WPOI alone was also significantly predictive for LRR adjusted for potential confounders with a cut-point of either WPOI-4 (p = 0.0029, HR 3.63, 95 % CI 1.56, 8.47) or WPOI-5 (p = 0.0008, HR 2.55, 95 % CI 1.48, 4.41) and for DSS (cut point WPOI-5, p = 0.0001, HR 6.34, 95 % CI 2.50, 16.09). Given a WPOI-5, the probability of developing locoregional recurrence is 42 %. Given a high-risk classification for a combination of features other than WPOI-5, the probability of developing locoregional recurrence is 32 %. The Risk Model is the first validated model that is significantly predictive for the important niche group of low-stage OCSCC patients.














Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Sessions DG, Spector GJ, Lenox J, et al. Analysis of treatment results for oral tongue cancer. Laryngoscope. 2002;112:616–25.
Jerjes W, Upile T, Hamdoon Z, et al. Prospective evaluation of outcome after transoral CO(2) laser resection of T1/T2 oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:180–7.
Huang TY, Hsu LP, Wen YH, et al. Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol. 2010;46:49–55.
Brandwein-Gensler M, Lewis C, Lee B, et al. Oral squamous cell carcinoma: histological risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;20:167–78.
Jakobsson PA, Eneroth CM, Killander D, et al. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973;12:1–8.
Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinoma. Scan J Dent Res. 1987;95:229–49.
Bryne M, Koppang HS, Lilleng R, et al. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432–7.
Bryne M, Koppang HS, Lilleng R, Kjaerheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375–81.
Bryne M, Jenssen N, Boysen M. Histologic grading in the deep invasive front of T1 and T2 glottic squamous cell carcinomas has high prognostic value. Virch Arch. 1995;427:277–81.
Brandwein-Gensler M, Smith RV, Wang B, et al. Validation of the histological Risk Model in a new patient cohort with primary head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676–88.
Vered M, Dayan D, Dobriyan A, et al. Oral tongue squamous cell carcinoma: recurrent disease is associated with histopathologic risk score and young age. J Cancer Res Clin Oncol. 2010;136:1039–48.
Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
Fine JP, Gray RJ. A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc. 1999;94:456–509.
Brennan S, Corry J, Kleid S, et al. Prospective trial to evaluate staged neck dissection or elective neck radiotherapy in patients with CT-staged T1–2 N0 squamous cell carcinoma of the oral tongue. Head Neck. 2010;32:191–8.
Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19:205–10.
Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in squamous carcinoma of tongue a prospective randomized trial. Am J Surg. 1989;158:309–13.
Sporano A, Weinstein G, Challian A, et al. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004;131:472–6.
Bundgaard T, Rossen K, Henriksen SD, et al. Histologic parameters in the evaluation of T1 squamous cell carcinomas of the oral cavity. Head Neck. 2002;24:656–60.
Odell EW, Jani P, Sherriff M, et al. The prognostic value of individual histologic grading parameters in small lingual squamous cell carcinomas. The importance of the pattern of invasion. Cancer. 1994;74:789–94.
Po WYA, Lam KY, Lam LK, et al. Prognostic factors of clini- cally stage I and II oral tongue carcinoma. A comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002;24:513–20.
Sawair FA, Irwin CR, Gordon DJ, et al. Invasive front grading: reliability and usefulness in the management of oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:1–9.
Warburton G, Nikitakis NG, Roberson P, et al. Histopathological and lymphangiogenic parameters in relation to lymph node metastasis in early stage oral squamous cell carcinoma. J Oral Maxillofac Surg. 2007;65:475–84.
Maleki S, Schlecht NF, Keller C, et al. Lymphocytic host response to oral squamous cell carcinoma: an adaptive T-cell response at the tumor interface. Head Neck Pathol. 2011;2:117–22.
Kawakita D, Hosono S, Ito H, et al. Impact of smoking status on clinical outcome in oral cavity cancer patients. Oral Oncol. 2012;48:186–91.
de Matos FR, Lima Ed, Queiroz LM, da Silveira EJ. Analysis of inflammatory infiltrate, perineural invasion, and risk score can indicate concurrent metastasis in squamous cell carcinoma of the tongue. J Oral Maxillofac Surg. 2012;70:1703–10.
Acknowledgments
We thank the following surgeons: Mark DeLacure MD, David Myssiorek MD, and Kepal Patel MD, from New York University Langone Medical Center, and Rick Nason MD from University of Manitoba, Cancer Care Manitoba, Winnipeg, Canada for access to their patient medical records. We also thank Cathy Sarta, RN, for meticulous patient follow-up for the Montefiore Medical Center/Albert Einstein College of Medicine cohort.
Author information
Authors and Affiliations
Corresponding author
Additional information
Both Drs. Negassa and Brandwein-Gensler are the senior authors.
Rights and permissions
About this article
Cite this article
Li, Y., Bai, S., Carroll, W. et al. Validation of the Risk Model: High-Risk Classification and Tumor Pattern of Invasion Predict Outcome for Patients with Low-Stage Oral Cavity Squamous Cell Carcinoma. Head and Neck Pathol 7, 211–223 (2013). https://doi.org/10.1007/s12105-012-0412-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-012-0412-1