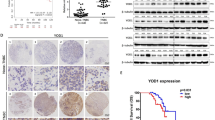
Abstract
Purpose
Despite significant improvement in therapeutic development in the past decades, breast cancer remains a formidable cause of death for women worldwide. The hormone positive subtype (HR(+)) (also known as luminal type) is the most prevalant category of breast cancer, comprising ~70% of patients. The clinical success of the three CDK4/6 inhibitors palbociclib, ribociclib, and abemaciclib has revolutionized the treatment of choice for metastatic HR(+) breast cancer. Accumulating evidence demonstrate that the properties of CDK4/6 inhibitors extend beyond inhibition of the cell cycle, including modulation of immune function, sensitizing PI3K inhibitors, metabolism reprogramming, kinome rewiring, modulation of the proteosome, and many others. The ubiquitin–proteasome pathway (UPP) is a crucial cellular proteolytic system that maintains the homeostasis and turnover of proteins.
Methods
We performed transcriptional profiling of the HR(+) breast cancer cell lines MCF7 and T47D treated with Palbociclib. Differential expressed genes were analyzed for novel pathways enriched. The results were further validated with biochemical assays and with real world clinical database cohorts.
Results
We uncovered a novel mechanism that demonstrate the CDK4/6 inhibitors suppress the expression of three ubiquitin conjugating enzymes UBE2C, UBE2S, UBE2T. Further validation in the HR(+) cell lines show that Palbociclib and ribociclib decrease UBE2C at both the mRNA and protein level, but this phenomenon was not shared with abemaciclib. These three E2 enzymes modulate several E3 ubiquitin ligases, including the APC/C complex which plays a role in G1/S progression. We further demonstrate the UBE2C/UBE2T expression levels are associated with breast cancer survival, and HR(+) breast cancer cells demonstrate dependence on the UBE2C.
Conclusions
Our study suggests a novel link between CDK4/6 inhibitor and UPP pathway, adding to the potential mechanisms of their clinical efficacy in cancer.










Similar content being viewed by others
Change history
10 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12094-022-02928-2
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Li Y, Yang D, Yin X, Zhang X, Huang J, Wu Y, et al. Clinicopathological characteristics and breast cancer-specific survival of patients with single hormone receptor-positive breast cancer. JAMA Netw Open. 2020;3(1):e1918160.
Chi D, Singhal H, Li L, Xiao T, Liu W, Pun M, et al. Estrogen receptor signaling is reprogrammed during breast tumorigenesis. Proc Natl Acad Sci USA. 2019;116(23):11437–43.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO International consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–49.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and Letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–48.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol off J Am Soc Clin Oncol. 2017;35(32):3638–46.
Knudsen ES, Shapiro GI, Keyomarsi K. Selective CDK4/6 inhibitors: biologic outcomes, determinants of sensitivity, mechanisms of resistance, combinatorial approaches, and pharmacodynamic biomarkers. Am Soc Clin Oncol Educ Book. 2020;40:115–26.
Goel S, DeCristo MJ, McAllister SS, Zhao JJ. CDK4/6 Inhibition in cancer: beyond cell cycle arrest. Trends Cell Biol. 2018;28(11):911–25.
Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548(7668):471–5.
Lelliott EJ, Kong IY, Zethoven M, Ramsbottom KM, Martelotto LG, Meyran D, et al. CDK4/6 inhibition promotes antitumor immunity through the induction of t-cell memory. Cancer Discov. 2021;11(10):2582–601.
Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26(1):136–49.
Franco J, Balaji U, Freinkman E, Witkiewicz AK, Knudsen ES. Metabolic reprogramming of pancreatic cancer mediated by CDK4/6 inhibition elicits unique vulnerabilities. Cell Rep. 2016;14(5):979–90.
Pancholi S, Ribas R, Simigdala N, Schuster E, Nikitorowicz-Buniak J, Ressa A, et al. Tumour kinome re-wiring governs resistance to palbociclib in oestrogen receptor positive breast cancers, highlighting new therapeutic modalities. Oncogene. 2020;39(25):4781–97.
Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 inhibitors: the mechanism of action may not be as simple as once thought. Cancer Cell. 2018;34(1):9–20.
Du Q, Guo X, Wang M, Li Y, Sun X, Li Q. The application and prospect of CDK4/6 inhibitors in malignant solid tumors. J Hematol Oncol. 2020;13(1):41.
Pernas S, Tolaney SM, Winer EP, Goel S. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol. 2018;10:1758835918786451.
Scheffner M, Nuber U, Huibregtse JM. Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature. 1995;373(6509):81–3.
Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochem Biophys Acta. 2004;1695(1–3):55–72.
George AJ, Hoffiz YC, Charles AJ, Zhu Y, Mabb AM. A Comprehensive atlas of E3 Ubiquitin ligase mutations in neurological disorders. Front Genet. 2018;9:29.
Miettinen TP, Peltier J, Hartlova A, Gierlinski M, Jansen VM, Trost M, et al. Thermal proteome profiling of breast cancer cells reveals proteasomal activation by CDK4/6 inhibitor palbociclib. EMBO J. 2018;37(10):e98359.
Kariri Y, Toss MS, Alsaleem M, Elsharawy KA, Joseph C, Mongan NP, et al. Ubiquitin-conjugating enzyme 2C (UBE2C) is a poor prognostic biomarker in invasive breast cancer. Breast Cancer Res Treat. 2022;192(3):529–39.
Shan BQ, Wang XM, Zheng L, Han Y, Gao J, Lv MD, et al. DCAF13 promotes breast cancer cell proliferation by ubiquitin inhibiting PERP expression. Cancer Sci. 2022;113(5):1589.
Huang W, Liu X, Zhang Y, Deng M, Li G, Chen G, et al. USP5 promotes breast cancer cell proliferation and metastasis by stabilizing HIF2alpha. J Cell Physiol. 2022;237(4):2211–9.
Howley BV, Mohanty B, Dalton A, Grelet S, Karam J, Dincman T, et al. The ubiquitin E3 ligase ARIH1 regulates hnRNP E1 protein stability EMT and breast cancer progression. Oncogene. 2022;41(12):1679–90.
Martinez-Chacin RC, Bodrug T, Bolhuis DL, Kedziora KM, Bonacci T, Ordureau A, et al. Ubiquitin chain-elongating enzyme UBE2S activates the RING E3 ligase APC/C for substrate priming. Nat Struct Mol Biol. 2020;27(6):550–60.
Liess AKL, Kucerova A, Schweimer K, Schlesinger D, Dybkov O, Urlaub H, et al. Dimerization regulates the human APC/C-associated ubiquitin-conjugating enzyme UBE2S. Sci Signal. 2020. https://doi.org/10.1126/scisignal.aba8208.
Kernan J, Bonacci T, Emanuele MJ. Who guards the guardian? mechanisms that restrain APC/C during the cell cycle. Biochim Biophys Acta Mol Cell Res. 2018;1865(12):1924–33.
Hu D, Qiao X, Wu G, Wan Y. The emerging role of APC/CCdh1 in development. Semin Cell Dev Biol. 2011;22(6):579–85.
da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57.
Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, et al. DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35(2):W169–75.
Lanczky A, Gyorffy B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J Med Internet Res. 2021;23(7):e27633.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98-102.
Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, et al. Defining a cancer dependency map. Cell. 2017;170(3):564-76.e16.
Wagner V, Gil J. Senescence as a therapeutically relevant response to CDK4/6 inhibitors. Oncogene. 2020;39(29):5165–76.
Crozier L, Foy R, Mouery BL, Whitaker RH, Corno A, Spanos C, et al. CDK4/6 inhibitors induce replication stress to cause long-term cell cycle withdrawal. EMBO J. 2022;41(6):e108599.
Zhang Z, Li J, Ou Y, Yang G, Deng K, Wang Q, et al. CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther. 2020;5(1):25.
Kuner R, Falth M, Pressinotti NC, Brase JC, Puig SB, Metzger J, et al. The maternal embryonic leucine zipper kinase (MELK) is upregulated in high-grade prostate cancer. J Mol Med (Berl). 2013;91(2):237–48.
O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–30.
Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571–81.
Pandey K, An HJ, Kim SK, Lee SA, Kim S, Lim SM, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int J Cancer. 2019;145(5):1179–88.
Wander SA, Cohen O, Gong X, Johnson GN, Buendia-Buendia JE, Lloyd MR, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 2020;10(8):1174–93.
Yeon Hee Park S-AI, Kyunghee Park, Ji Wen, Ahrum Min, Vinicius Bonato, Seri Park, Sripad Ram, Dae-Won Lee, Ji-Yeon Kim, Kyung-Hun Lee, Won-Chul Lee, Jisook Lee, Hyunseon(Ally) Kim, Won-Woo Lee, Yoon-La Choi, Scott Weinrich, Han Suk Ryu, Woong-Yang Park, Zhengyan Kan, Palbociclib Biomarker Translational Research Group (Palbo-Bio-TRG). Prospective longitudinal multi-omics study of palbociclib resistance in hormone receptor+/HER2- metastatic breast cancer. ASCO Annu Meet. 2021;39(15):1013–23.
Klein FG, Granier C, Zhao Y, Pan Q, Tong Z, Gschwend JE, et al. Combination of Talazoparib and Palbociclib as a potent treatment strategy in bladder cancer. J Pers Med. 2021;11(5):340.
Li S, Zhang Y, Wang N, Guo R, Liu Q, Lv C, et al. Pan-cancer analysis reveals synergistic effects of CDK4/6i and PARPi combination treatment in RB-proficient and RB-deficient breast cancer cells. Cell Death Dis. 2020;11(4):219.
Brinkmann K, Schell M, Hoppe T, Kashkar H. Regulation of the DNA damage response by ubiquitin conjugation. Front Genet. 2015;6:98.
Jacquemont C, Taniguchi T. Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Can Res. 2007;67(15):7395–405.
Marra A, Curigliano G. Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer. 2019;5:27.
Pham HT, Nguyen TTT, Nguyen LP, Han SS, Lim YS, Hwang SB. Hepatitis C virus downregulates ubiquitin-conjugating enzyme e2s expression to prevent proteasomal degradation of NS5A, leading to host cells more sensitive to DNA damage. J Virol. 2019;93(2):e01240-e1318.
Sacaan AI, Thibault S, Hong M, Kondegowda NG, Nichols T, Li R, et al. CDK4/6 inhibition on glucose and pancreatic beta cell homeostasis in young and aged rats. Mol Cancer Res. 2017;15(11):1531–41.
Liu Y, Zhao R, Chi S, Zhang W, Xiao C, Zhou X, et al. UBE2C Is upregulated by estrogen and promotes epithelial-mesenchymal transition via p53 in endometrial cancer. Mol Cancer Res. 2020;18(2):204–15.
Min M, Mevissen TE, De Luca M, Komander D, Lindon C. Efficient APC/C substrate degradation in cells undergoing mitotic exit depends on K11 ubiquitin linkages. Mol Biol Cell. 2015;26(24):4325–32.
Watson ER, Brown NG, Peters JM, Stark H, Schulman BA. Posing the APC/C E3 ubiquitin ligase to orchestrate cell division. Trends Cell Biol. 2019;29(2):117–34.
Mizrak A, Morgan DO. Polyanions provide selective control of APC/C interactions with the activator subunit. Nat Commun. 2019;10(1):5807.
Binne UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG Jr, et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol. 2007;9(2):225–32.
Mo CH, Gao L, Zhu XF, Wei KL, Zeng JJ, Chen G, et al. The clinicopathological significance of UBE2C in breast cancer: a study based on immunohistochemistry, microarray and RNA-sequencing data. Cancer Cell Int. 2017;17:83.
Psyrri A, Kalogeras KT, Kronenwett R, Wirtz RM, Batistatou A, Bournakis E, et al. Prognostic significance of UBE2C mRNA expression in high-risk early breast cancer a Hellenic Cooperative Oncology Group (HeCOG) Study. Ann Oncol. 2012;23(6):1422–7.
Bavi P, Uddin S, Ahmed M, Jehan Z, Bu R, Abubaker J, et al. Bortezomib stabilizes mitotic cyclins and prevents cell cycle progression via inhibition of UBE2C in colorectal carcinoma. Am J Pathol. 2011;178(5):2109–20.
Wang H, Zhang C, Rorick A, Wu D, Chiu M, Thomas-Ahner J, et al. CCI-779 inhibits cell-cycle G2-M progression and invasion of castration-resistant prostate cancer via attenuation of UBE2C transcription and mRNA stability. Can Res. 2011;71(14):4866–76.
Kim YJ, Lee G, Han J, Song K, Choi JS, Choi YL, et al. UBE2C overexpression aggravates patient outcome by promoting estrogen-dependent/independent cell proliferation in early hormone receptor-positive and HER2-negative breast cancer. Front Oncol. 2019;9:1574.
Acknowledgements
This study was partially funded by the grants to JIL: MOST 110-2314-B-A49A-549 (Ministry of Science and Technology), MLCF-V111_A11102 (Melissa Lee Cancer Foundation), Taiwan Clinical Oncology Research Foundation, and 109DHA0100490 (Taipei Veterans General Hospital internal grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to structure the Abstract and to correct the formatting of the Results section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreementwith the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, CY., Yu, CJ., Liu, CY. et al. CDK4/6 inhibitors downregulate the ubiquitin-conjugating enzymes UBE2C/S/T involved in the ubiquitin–proteasome pathway in ER + breast cancer. Clin Transl Oncol 24, 2120–2135 (2022). https://doi.org/10.1007/s12094-022-02881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-02881-0