Abstract
Purpose
To study the imaging parameters of 18F-fluorodeoxy glucose (18F-FDG) in breast cancer on positron emission tomography/computed tomography (PET/CT)—the correlation of clinical pathological factors and prognosis among the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of lesions for patients.
Methods
From January 2012 to December 2014, a total of 125 female patients were treated in our hospital for the first time and were diagnosed as breast cancer by histopathology. They were selected as the research subjects. All of them had complete 18F-FDG PET/CT examination data before surgery, the postoperative clinicopathological information, and follow-up data. They were divided into the event group (38 cases) and the event-free group (87 cases) according to whether local recurrence or distant metastasis occurred after the follow-up, with the follow-up time 4–60 months. The correlation on 18F-FDG PET/CT metabolic parameters of breast cancer with clinicopathological factors and prognosis was retrospectively evaluated.
Results
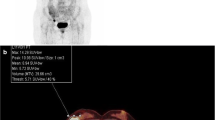
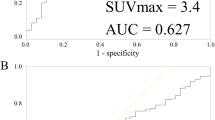
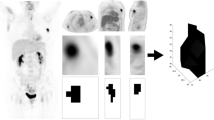
The primary lesions of 125 cases with breast cancers all had higher 18F-FDG uptake, and the SUVmax, MTV, and TLG of the primary tumors in the event group were significantly higher than those in the event-free group (t = 2.645, 2.782, 15.263, p = 0.011, 0.008, 0.000), p < 0.05; SUVmax, MTV, and TLG of primary breast cancer have no correlation with age and tumor site of patient (p > 0.05); there were statistically significant differences in the SUVmax, MTV, and TLG of primary tumor in the comparison of different tumor size, T stage, N stage, and histological grades (p < 0.05); all of SUVmax, MTV, and TLG in the estrogen receptor (ER) and/or progesterone receptor (PR) positive groups were lower than those in the negative group, with statistically significant difference (p < 0.05); the SUVmax, MTV, and TLG of human epidermal growth factor receptor 2 (HER2) positive group, proliferating cell nuclear antigen (Ki-67) high expression group were higher than those in the negative group and low expression group, with statistically significant difference (p < 0.05). There were 38 recurrence and metastasis cases within 125 cases with breast cancer in 5 years after operation, with the total recurrence and metastasis rate as 30.40% (38/125). The event-free survival rate in the SUVmax ≥ 8.64 group was significantly lower than that in the SUVmax < 8.64 group (p < 0.01).
Conclusions
The metabolic parameters of 18F-FDG PET/CT in breast cancer can reflect the biological behavior of the tumor indirectly; therefore, it was studied on the related correlation to provide the guidance of clinical individualized comprehensive treatment and prognostic judgment.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Bevers TB, Anderson BO, Bonaccio E, Buys S, Daly MB, Dempsey PJ, Farrar WB, Fleming I, Garber JE, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Cancer Netw. 2009;7:1060–96.
Groheux D, Hindié E, Giacchetti S, Hamy AS, Berger F, Merlet P, de Roquancourt A, de Cremoux P, Marty M, Hatt M, Espié M. Early assessment with 18F-fluorodeoxyglucose positron emission tomography/computed tomography can help predict the outcome of neoadjuvant chemotherapy in triple negative breast cancer. Eur J Cancer. 2014;50:1864–71.
Piva R, Ticconi F, Ceriani V, Scalorbi F, Fiz F, Capitanio S, Bauckneht M, Cittadini G, Sambuceti G, Morbelli S. Comparative diagnostic accuracy of 18F-FDG PET/CT for breast cancer recurrence. Breast Cancer (Dove Med Press). 2017;9:461–71.
Ege Aktas G, Taştekin E, Sarikaya A. Assessment of biological and clinical aggressiveness of invasive ductal breast cancer using baseline 18F-FDG PET/CT-derived volumetric parameters. Nucl Med Commun. 2018;39:83–93.
Groheux D, Majdoub M, Tixier F, Le Rest CC, Martineau A, Merlet P, Espié M, de Roquancourt A, Hindié E, Hatt M, Visvikis D. Do clinical, histological or immunohistochemical primary tumour characteristics translate into different (18)F-FDG PET/CT volumetric and heterogeneity features in stage II/III breast cancer? Eur J Nucl Med Mol Imaging. 2015;42:1682–91.
Kim BS, Sung SH. Usefulness of 18F-FDG uptake with clinicopathologic and immunohistochemical prognostic factors in breast cancer. Ann Nucl Med. 2012;26:175–83.
Sanli Y, Kuyumcu S, Ozkan ZG, Işik G, Karanlik H, Guzelbey B, Turkmen C, Ozel S, Yavuz E, Mudun A. Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med. 2012;26:345–50.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Kim YH, Yoon HJ, Kim Y, Kim BS. Axillary lymph node-to-primary tumor standard uptake value ratio on preoperative (18)F-FDGPET/CT: a prognostic factor for invasive ductal breast cancer. J Breast Cancer. 2015;18:173–80.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, Panel members. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47.
Ulaner GA, Castillo R, Goldman DA, Wills J, Riedl CC, Pinker-Domenig K, Jochelson MS, Gönen M. (18)F-FDG-PET/CT for systemic staging of newly diagnosed triple-negative breast cancer. Eur J Nucl Med Mol Imaging. 2016;43:1937–44.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, Yamamoto M, Hama Y, Tamura K, Ishida J, Abe Y, Mochizuki H. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucosepositron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8.
Chen S, Ibrahim NK, Yan Y, Wong ST, Wang H, Wong FC. Risk stratification in patients with advanced-stage breast cancer by pretreatment [(18) F]FDG PET/CT. Cancer. 2015;121:3965–74.
Kajáry K, Tőkés T, Dank M, Kulka J, Szakáll S Jr, Lengyel Z. Correlation of the value of 18F-FDG uptake, described by SUVmax, SUVavg, metabolic tumour volume and total lesion glycolysis, to clinicopathological prognostic factors and biological subtypes in breast cancer. Nucl Med Commun. 2015;36:28–37.
Kaida H, Toh U, Hayakawa M, Hattori S, Fujii T, Kurata S, Kawahara A, Hirose Y, Kage M, Ishibashi M. The relationship between 18F-FDG metabolic volumetric parameters and clinicopathological factors of breast cancer. Nucl Med Commun. 2013;34:562–70.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–23.
Dawood S, Hu R, Homes MD, Collins LC, Schnitt SJ, Connolly J, Colditz GA, Tamimi RM. Defining breast cancer prognosis based on molecular phenotypes: results from a large cohort study. Breast Cancer Res Treat. 2011;126:185–92.
Song B, Wang L, Zhang Y, Li N, Dai H, Xu H, Cai H, Yan J. Combined detection of HER2, Ki67, and GSTP1 genes on the diagnosis and prognosis of breast cancer. Cancer Biother Radiopharm. 2019;34:85–90.
García Fernández A, Chabrera C, García Font M, Fraile M, Lain JM, Gónzalez S, Barco I, González C, Torres J, Piqueras M, Cirera L, Veloso E. Differential patterns of recurrence and specific survival between luminal A and luminal B breast cancer according to recent changes in the 2013 St Gallen immunohistochemical classification. Clin Transl Oncol. 2015;17:238–46.
Elkablawy MA, Albasri AM, Mohammed RA, Hussainy AS, Nouh MM, Alhujaily AS. Ki67 expression in breast cancer. Correlation with prognostic markers and clinicopathological parameters in Saudi patients. Saudi Med J. 2016;37:137–41.
Chen X, He C, Han D, Zhou M, Wang Q, Tian J, Li L, Xu F, Zhou E, Yang K. The predictive value of Ki-67 before neoadjuvant chemotherapy for breast cancer: a systematic review and meta-analysis. Future Oncol. 2017;13:843–57.
Tchou J, Sonnad SS, Bergey MR, Basu S, Tomaszewski J, Alavi A, Schnall M. Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol Imaging Biol. 2010;12:657–62.
Tőkés T, Somlai K, Székely B, Kulka J, Szentmártoni G, Torgyík L, Galgóczy H, Lengyel Z, Györke T, Dank M. The role of FDG-PET-CT in the evaluation of primary systemic therapy in breast cancer: links between metabolic and pathological remission. Orv Hetil. 2012;153:1958–64.
Jiménez-Ballvé A, García García-Esquinas M, Salsidua-Arroyo O, Serrano-Palacio A, García-Sáenz JA, Ortega Candil A, Fuentes Ferrer ME, Rodríguez Rey C, Román-Santamaría JM, Moreno F, Carreras-Delgado JL. Prognostic value of metabolic tumour volume and total lesion glycolysis in 18F-FDG PET/CTscans in locally advanced breast cancer staging. Rev Esp Med Nucl Imagen Mol. 2016;35:365–72.
Groheux D, Martineau A, Teixeira L, Espié M, de Cremoux P, Bertheau P, Merlet P, Lemarignier C. 18FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. 2017;19:3.
Funding
This work was supported by Shandong Medical and Health Technology Development Plan Project (no. 2017WSB832), Shandong Provincial Higher Education Research and Development Program (no. J18KB069).
Author information
Authors and Affiliations
Contributions
Y-HQ designed the study and drafted the manuscript. NL and CR were responsible for the collection and analysis of the experimental data. JS revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by Ethical Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao University and conducted in accordance with the ethical standards.
Informed consent
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, YH., Long, N., Ran, C. et al. The correlation of 18F-FDG PET/CT metabolic parameters, clinicopathological factors, and prognosis in breast cancer. Clin Transl Oncol 23, 620–627 (2021). https://doi.org/10.1007/s12094-020-02457-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02457-w