Abstract
Background
Promising results have been reported with immune checkpoint inhibitors (ICI) in a small proportion of MPM patients. MMR deficiency (dMMR) has been well described in several malignancies and was approved as a biomarker for anti-PD-1 inhibitors. Next generation sequencing (NGS) data demonstrated that 2% of MPM harbor microsatellite instability. The aim of this study is to characterize MMR by immunohistochemistry (IHC) in a series of MPM including a subset of patients treated with immunotherapy.
Methods
Tumors of 159 MPM p diagnosed between 2002 and 2017 were reviewed. Formalin-fixed, paraffin-embedded tissue was stained for MLH1, MSH2, MSH6 and PMS2 and tumors were classified as dMMR (MMR protein expression negative) and MMR intact (all MMR proteins positively expressed). We retrospectively collected clinical outcomes under standard chemotherapy and experimental immunotherapy in the entire cohort.
Results
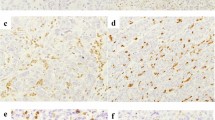
MMR protein expression was analyzed in 158 samples with enough tissue and was positive in all of the cases. Twenty two patients received ICI with anti-CTLA4 or anti-PD-1 blockade in clinical trials, 58% had a response or stable disease for more than 6 m, with median progression-free survival (PFS) of 5.7 m (2.1–26.1 m). The median overall survival (mOS) in all population was 15 months (m) (13.5–18.8 m). In a multivariable model factors associated to improved mOS were PS 0, neutrophil–lymphocyte ratio (NLR) < 5 and epithelioid histology (p < 0.001).
Conclusions
In our series we were unable to identify any MPM patient with dMMR by IHC. Further studies are needed to elucidate potential predictive biomarkers of ICI benefit in MPM.



Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Tsao A, Lindwasser O, Adjei A, Adusimilli P, Beyers M, Blumenthal G, et al. Current and future management of malignant mesothelioma: a consensus report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and mesothelioma applied research foundation. J Thorac Oncol. 2018;11:1655–67.
Galateau-Salle F, Churg A, Roggli V, Travis W, On behalf of the World Health Organization Committee for tumors of pleura. The 2015 Worl Health Organization classification of tumors of the pleural: advances since the 2004 classification. J Thorac Oncol. 2015;11(2):142–54.
Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–16.
Hmeljak J, Sanchez-Vega F, Hoadley K, Shih J, Stewart C, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8(12):1548–65.
Maio M, Scherpereel A, Calabro L, Aerts J, Cedres S, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international. Randomized, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017:18(9):1261–1273
Alley E, Lopez J, Santoro A, Morosky A, Saraf S, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised open-label, phase 1b trial. Lancet Oncol. 2017;18(5):623–30.
Desai A, Karrison T, Rose B, Pemberton E, Hill B, et al. A phase II trial of pembrolizumab in patients with previously-treated mesothelioma (MM). J Clin Oncol. 2018;36:8565–856.
Popat S, Curioni-Fontecedro A, Polydoropoulou V, Shah R, O´Brien M, et al. A multicentre randomized pase III trial comparing pembrolizumab vs single agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: results from the European Thoracic Oncology Platform (ETOP9-15) PROMISE-meso trial. Ann Oncol. 2019;30:5.
Scherpereel A, Greiller L, Lantuejoul S, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501MAPS2): a multicenter, open-label, randomized, non-comparative, phase 2 trial. Lancet Oncol. 2019;20(239):253.
Disselhorst M, Quispel-Janssen J, Lalezani F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single arm, phase 2 trial. Lancet Resp Med. 2019. https://doi.org/10.1016/S2213-2600(18)30420-X.
Okada M, Tm K, Aoe K, Kato T, Fujimoto N, et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open label, single arm, Japanese phase II study in malignant pleural mesothelioma (MERIT). Clin Cancer Res. 2019;25:5485–92.
Teng F, Meng X, Kong L, Yu J. Progress and challenges of predictive biomarkers of antiPD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166–73.
Camidge D, Doebele R, Kerr K. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol. 2019. https://doi.org/10.1038/s41571-019-0173-9.
Cedres S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death ligand (PD-L1) in malignant pleural mesothelioma (MPM). PLoS ONE. 2015;16:e0121071.
Mansfield A, Roden A, Peikert T, Sheinin YM, Harrington SM, Krco CJ, et al. B7–H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036–40.
Rivalland G, Chuan-Hao Kao S, Pavlakis N, et al. Outcomes ofanti-PD-1 therapy in mesothelioma and correlation with PD-L1 expression. Proc Am Soc Clin Oncol. 2017;35:8514.
Quispel-Janssen J, Zago G, Schouten R, Buikhuisen W, et al. A phase II study of nivolumab in malignant pleural mesothelioma (NivoMes): with translational research (TR) biopsies. J Thorac Oncol. 2017;12:S292–S293293.
Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75:264–269.
Mansfield AS, Peikert T, Smadbeck JB, et al. Neoantigenic potential of complex chromosomal rearrangements in mesothelioma. J Thorac Oncol. 2019;14:276–87.
Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park P. A molecular portrait of microsatellite instability across multiple cancers. Nature communications. 2017;8:15180. https://doi.org/10.1038/ncomms15180.
Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–46.
Iino H, Simms L, Young J, Arnold J, Winship IM, Webb SI, Furlong KL, Leggett B, Jass JR. DNA microsatellite instability and mismatch repair protein loss in adenomas presenting in hereditary nonpolyposis colorectal cancer. Gut. 2000;47:37–42.
Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, La Jeunesse J, Nakagawa H, Westman JA, Prior TW, Clendenning M, Penzone P, Lombardi J, Dunn P, Cohn DE, Copeland L, Eaton L, Fowler J, Lewandowski G, Vaccarello L, Bell J, Reid G, de la Chapelle A. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2006;66:7810–7.
Zhu L, Wang Y, Zhang C, Liu Y, Qu X. microsatellite instability and survival in gastric cancer: a systematic review and meta-analysis. Mol Clin Oncol. 2015;3:699–705.
Cicek MS, Lindor NM, Gallinger S, Bapat B, Hopper JL, Jenkins MA, Young J, Buchanan D, Walsh MD, Le Marchand L, et al. Quality assessment and correlation of microsatellite instability and immunohistochemical markers among population- and clinic-based colorectal tumors results from the Colon Cancer Family Registry. J Mol Diagn. 2011;13:271–81.
Salipante SJ, Scroggins SM, Hampel HL, et al. Microsatellite instability detection by next generation sequencing. Clin Chem. 2014;60:1192–9.
Niu B, Ye K, Zhang Q, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2013;30:1015–6.
Kautto EA, Bonneville R, Miya J, et al. Performance evaluation for rapid detection of pan-cancer microsatellite instability with MANTIS. Oncotarget. 2016;8:7452–63.
Bonneville R, Krook M, Kautto E, Miya J, Wing M, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017. https://doi.org/10.1200/PO17.00073.
Le D, Uram J, Wang H, Barlett B, Kemberling H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20.
Arulananda S, Thapa B, Walkiewicz M, et al. Mismatch repair protein defects and microsatellite instability in malignant pleural mesothelioma. J Thorac Oncol. 2018;13(10):1588–94.
Rami-Porta R. Staging manual in thoracic oncology from inetrnational association for the study of lung cancer. ISBN:978–0–9832958–4–6
Luchini C, Bibeau F, Ligtenberg J, Singh N, Nottegar A, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30:1232–43.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controll Clin Trials. 1996;17(4):343–6.
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57.
Kim GP, Colangelo LH, Wieand HS, Paik S, Kirsch IR, Wolmark N, Allegra CJ. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25(7):767–72.
Kim C, Ahn J, Jung M, Beom S, Kim C, Kim J, et al. effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br J Cancer. 2016;115:25–33.
Overman M, McDermott R, Lleach J, Lonardi S, Lenz H, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91.
Marginean E, Melosky B. Is there a role for programmed death ligand-1 testing and immunotherapy in colorectal cancer with microsatellite instability? Arch Pathol Lab Med. 2018;142:17–25.
Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal Cancer patients at risk for hereditary nonpolyposis colorectal Cancer syndrome. J Mol Diagn. 2008;10:293–300.
Modica I, Soslow RA, Black D, Tornos C, Kauff N, Shia J. Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol. 2007;31:744–51.
Wimmer K, Krtaz C, Vasen H, Caron O, Colas C, et al. Diagnostic criterai for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium “Care for CMMRD” (C4CMMRD). J Med Genetic. 2014;51:355–65.
Bodo S, Colas C, Buhard O, Collura A, Tinat J, et al. Diagnosis of constitutional mismatch repair-deficiency syndrome based on microsatellite instability and lymphocyte tolerance to methylating agents. Gastroenterology. 2015;149:1017–29.
Giannakis M, Mu X, Shukla S, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15:857–65.
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8.
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99.
Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44.
Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–14.
Acknowledgements
We want to acknowledge the Cellex Foundation for providing facilities and equipment, and Spanish Cancer Association (AECC) for its support.
Funding
This work was supported by the Spanish Cancer Association (AECC) Scientific Foundation (GCB14-2170).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Approval for the use of the tissue in research was obtained from the ethical local committee from both hospitals.
Informed consent
All patients gave written inform consent before enrollment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cedrés, S., Ponce-Aix, S., Iranzo, P. et al. Analysis of mismatch repair (MMR) proteins expression in a series of malignant pleural mesothelioma (MPM) patients. Clin Transl Oncol 22, 1390–1398 (2020). https://doi.org/10.1007/s12094-019-02275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02275-9