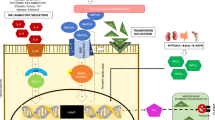
Abstract
Iron has a pivotal role in homeostasis due to its participation in virtually all of the body’s oxidationreduction processes. However, iron can also be considered a double-edged weapon, as its excess may lead to an increased risk of developing cancer, presumably by the generation of reactive oxygen species, and its role as substrate to enzymes that participate in cell proliferation. Thus, iron might as well be considered a cofactor in tumour cell proliferation. In certain pathological conditions, such as haemochromatosis, hepatitis B and C virus infection, asbestosis and endometriosis, iron overload may increase the risk of cancer. By contrast, iron depletion could be considered a useful adjunct in antitumour therapy. This paper reviews the current scientific evidence behind iron’s role as a protumoral agent, and the potential benefit of a state of iron depletion in patients with cancer.
Similar content being viewed by others
References
Zacharski LR, Ornstein DL, Woloshin S et al (2000) Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J 140:98–104
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Toyokuni S (2009) Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci 100:9–16
Crosby WH (1991) A history of phlebotomy therapy for hemochromatosis. Am J Med Sci 301:28–31
Cappellini MD (2005) Iron-chelating therapy with the new oral agent ICL670 (Exjade). Best Pract Res Clin Haematol 18:289–298
Darnell G, Richardson DR (1999) The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents III: the effect of the ligands on molecular targets involved in proliferation. Blood 94:781–792
Gao J, Lovejoy D, Richardson DR (1999) Effect of iron chelators with potent anti-proliferative activity on the expression of molecules involved in cell cycle progression and growth. Redox Rep 4:311–312
Kwok JC, Richardson DR (2002) The iron metabolism of neoplastic cells: alterations that facilitate proliferation? Crit Rev Oncol Hematol 42:65–78
Andrews NC, Bridges KR (1998) Disorders of iron metabolism and sideroblastic anemia. In: Nathan DG, Orkin SH (eds) Hematology of infancy and childhood, 5th Edn. WB Saunders, Philadelphia, p 423–429
Hentze MW, Muckenthaler MU, Galy B et al (2010) Two to tango: regulation of Mammalian iron metabolism. Cell 142:24–38
Frazer DM, Anderson GJ (2003) The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Mol Dis 30:288–297
Wulfert M, Kupper AC, Tapprich C et al (2008) Analysis of mitochondrial DNA in 104 patients with myelodysplastic syndromes. Exp Hematol 36:577–586
Klausner RD, Rouault TA, Harford JB (1993) Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 72:19–28
Casey JL, Hentze MW, Koeller DM et al (1988) Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science 240:924–928
Leibold EA, Munro HN (1988) Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5′ untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A 85:2171–2175
Hentze MW, Kuhn LC (1996) Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci U S A 93:8175–8182
Merkofer M, Kissner R, Hider RC et al (2006) Fenton chemistry and iron chelation under physiologically relevant conditions: electrochemistry and kinetics. Chem Res Toxicol 19:1263–1269
Barbouti A, Doulias PT, Zhu BZ et al (2001) Intracellular iron, but not copper, plays a critical role in hydrogen peroxide-induced DNA damage. Free Radic Biol Med 31:490–498
Pantopoulos K (2004) Iron metabolism and the IRE/IRP regulatory system: an update. Ann N Y Acad Sci 1012:1–13
McCord JM (2004) Iron, free radicals, and oxidative injury. J Nutr 134:3171S–3172S
Dizdaroglu M, Jaruga P, Birincioglu M et al (2002) Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32:1102–1115
Galaris D, Skiada V, Barbouti A (2008) Redox signaling and cancer: the role of “labile” iron. Cancer Lett 266:21–2
Koptyra M, Falinski R, Nowicki MO et al (2006) BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood 108:319–327
Kovacevic Z, Richardson DR (2006) The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis 27:2355–2366
Sanz G, Nomdedeu B, Such E et al (2008) Independent impact of iron overload and transfusion dependency on survival and leukemic evolution in patients with myelodysplastic syndrome. Blood (ASH Annual Meeting Abstracts) 112
Mainous AG 3rd, Gill JM, Carek PJ (2004) Elevated serum transferrin saturation and mortality. Ann Fam Med 2:133–138
Knekt P, Reunanen A, Takkunen H et al (1994) Body iron stores and risk of cancer. Int J Cancer 56:379–382
Mainous AG 3rd, Gill JM, Everett CJ (2005) Transferrin saturation, dietary iron intake, and risk of cancer. Ann Fam Med 3:131–137
Yoshinari K, Yuasa K, Iga F et al (1989) A growthpromoting factor for human myeloid leukemia cells from horse serum identified as horse serum transferrin. Biochim Biophys Acta 1010:28–34
Sekyere EO, Dunn LL, Rahmanto YS et al (2006) Role of melanotransferrin in iron metabolism: studies using targeted gene disruption in vivo. Blood 107:2599–2601
Richardson DR, Baker E (1990) The uptake of iron and transferrin by the human malignant melanoma cell. Biochim Biophys Acta 1053:1–12
Sekyere E, Richardson DR (2000) The membrane-bound transferrin homologue melanotransferrin: roles other than iron transport? FEBS Lett 483:11–16
Kawabata H, Germain RS, Vuong PT et al (2000) Transferrin receptor 2-alpha supports cell growth both in iron-chelated cultured cells and in vivo. J Biol Chem 275:16618–16625
Macedo MF, de Sousa M (2008) Transferrin and the transferrin receptor: of magic bullets and other concerns. Inflamm Allergy Drug Targets 7:41–52
Das Gupta A, Patil J, Shah VI (1996) Transferrin receptor expression by blast cells in acute lymphoblastic leukemia correlates with white cell count & immunophenotype. Indian J Med Res 104:226–233
Goding JW, Burns GF (1981) Monoclonal antibody OKT-9 recognizes the receptor for transferrin on human acute lymphocytic leukemia cells. J Immunol 127:1256–1258
Nouri AM, Smith S, Oliver TR et al (1998) Comparative expression of major histocompatibility complex (MHC) antigens on CD5+ and CD5-B cells in patients with chronic lymphocytic leukaemia (CLL). Eur J Cancer 34:1618–1622
Smilevska T, Stamatopoulos K, Samara M et al (2006) Transferrin receptor-1 and 2 expression in chronic lymphocytic leukemia. Leuk Res 30:183–189
Tei I, Makino Y, Sakagami H et al (1982) Decrease of transferrin receptor during mouse myeloid leukemia (M1) cell differentiation. Biochem Biophys Res Commun 107:1419–1424
Taetle R, Rhyner K, Castagnola J et al (1985) Role of transferrin, Fe, and transferrin receptors in myeloid leukemia cell growth. Studies with an antitransferrin receptor monoclonal antibody. J Clin Invest 75:1061–1067
Lepelletier Y, Camara-Clayette V, Jin H et al (2007) Prevention of mantle lymphoma tumor establishment by routing transferrin receptor toward lysosomal compartments. Cancer Res 67:1145–1154
Callens C, Moura IC, Lepelletier Y et al (2008) Recent advances in adult T-cell leukemia therapy: focus on a new anti-transferrin receptor monoclonal antibody. Leukemia 22:42–48
Habeshaw JA, Lister TA, Stansfeld AG et al (1983) Correlation of transferrin receptor expression with histological class and outcome in non-Hodgkin lymphoma. Lancet 1:498–501
Nejmeddine F, Raphael M, Martin A et al (1999) 67Ga scintigraphy in B-cell non-Hodgkin’s lymphoma: correlation of 67Ga uptake with histology and transferrin receptor expression. J Nucl Med 40:40–45
Deiss A (1983) Iron metabolism in reticuloendothelial cells. Semin Hematol 20:81–90
Ruggeri G, Iacobello C, Albertini A et al (1984) Studies of human ferritin in tissues and body fluids. Elsevier, Amsterdam
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275:161–203
Broxmeyer HE, Gentile P, Bognacki J et al (1983) Lactoferrin, transferrin and acidic isoferritins: regulatory molecules with potential therapeutic value in leukemia. Blood Cells 9:83–105
de Sousa M, Smithyman A, Tan C (1978) Suggested models of ecotaxopathy in lymphoreticular malignancy. A role for iron-binding proteins in the control of lymphoid cell migration. Am J Pathol 90:497–520
Hancock BW, Bruce L, May K et al (1979) Ferritin, a sensitizing substance in the leucocyte migration inhibition test in patients with malignant lymphoma. Br J Haematol 43:223–233
Aulbert E, Schmidt CG (1985) Ferritin: a tumor marker in myeloid leukemia. Cancer Detect Prev 8:297–302
Lang JM, Eber M, Methlin G et al (1982) Serum ferritin during the course of chronic myeloid leukemia. Increase of serum ferritin as a marker of dyserythropoiesis. Acta Haematol 67:145–149
Richardson DR, Ponka P (1994) The iron metabolism of the human neuroblastoma cell: lack of relationship between the efficacy of iron chelation and the inhibition of DNA synthesis. J Lab Clin Med 124:660–671
Gray CP, Franco AV, Arosio P et al (2001) Immunosuppressive effects of melanoma-derived heavy-chain ferritin are dependent on stimulation of IL-10 production. Int J Cancer 92:843–850
Kalinowski DS, Richardson DR (2005) The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol Rev 57:547–583
Epsztejn S, Glickstein H, Picard V et al (1999) H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood 94:3593–3603
Thelander L, Reichard P (1979) Reduction of ribonucleotides. Annu Rev Biochem 48:133–158
Renton FJ, Jeitner TM (1996) Cell cycle-dependent inhibition of the proliferation of human neural tumor cell lines by iron chelators. Biochem Pharmacol 51:1553–1561
Yu Y, Kovacevic Z, Richardson DR (2007) Tuning cell cycle regulation with an iron key. Cell Cycle 6:1982–1994
Leitch HA, Wong DH, Leger CS (2007) Improved survival in myelodysplastic syndromes patients receiving iron chelation therapy. Leuk Res 31[Suppl 1]:S15
Donfrancesco A, Deb G, Dominici C et al (1990) Effects of a single course of deferoxamine in neuroblastoma patients. Cancer Res 50:4929–4930
Estrov Z, Tawa A, Wang XH et al (1987) In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood 69:757–761
Kaplinsky C, Estrov Z, Freedman MH et al (1987) Effect of deferoxamine on DNA synthesis, DNA repair, cell proliferation, and differentiation of HL-60 cells. Leukemia 1:437–441
Whitnall M, Howard J, Ponka P et al (2006) A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci U S A 103:14901–14906
Gojo I, Tidwell ML, Greer J et al (2007) Phase I and pharmacokinetic study of Triapine, a potent ribonucleotide reductase inhibitor, in adults with advanced hematologic malignancies. Leuk Res 31:1165–1173
Richardson DR, Milnes K (1997) The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents II: the mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood 89:3025–3038
Chantrel-Groussard K, Gaboriau F, Pasdeloup N et al (2006) The new orally active iron chelator ICL670A exhibits a higher antiproliferative effect in human hepatocyte cultures than O-trensox. Eur J Pharmacol 541:129–137
Lescoat G, Chantrel-Groussard K, Pasdeloup N et al (2007) Antiproliferative and apoptotic effects in rat and human hepatoma cell cultures of the orally active iron chelator ICL670 compared to CP20: a possible relationship with polyamine metabolism. Cell Prolif 40:755–767
Schwartz RN (2007) Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm 64:S5–13; quiz S28–30
Weiss G, Wachter H, Fuchs D (1995) Linkage of cell-mediated immunity to iron metabolism. Immunol Today 16:495–500
Glaspy J, Crawford J, Vansteenkiste J et al (2010) Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 102:301–315
Farrell F, Lee A (2004) The erythropoietin receptor and its expression in tumor cells and other tissues. Oncologist 9[Suppl 5]:18–30
Sytkowski AJ (2007) Does erythropoietin have a dark side? Epo signaling and cancer cells. Sci STKE 2007:pe38
Sulkowska M, Wincewicz A, Chabowska A et al (2006) To give or not to give recombinant EPO to anemia endangered cancer patients. Prague Med Rep 107:281–289
Sinclair AM, Coxon A, McCaffery I et al (2010) Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood 115:4264–4272
Swift S, Ellison AR, Kassner P et al (2010) Absence of functional EpoR expression in human tumor cell lines. Blood 115:4254–4263
Beguin Y (2005) Intravenous iron and recombinant human erythropoietin in cancer patients. J Clin Oncol 23:651–652; author reply 652–653
Osterborg A, Steegmann JL, Hellmann A et al (2007) Phase II study of three dose levels of continuous erythropoietin receptor activator (C.E.R.A.) in anaemic patients with aggressive non-Hodgkin’s lymphoma receiving combination chemotherapy. Br J Haematol 136:736–744
de Witte T (2008) The role of iron in patients after bone marrow transplantation. Blood Rev 22[Suppl 2]:S22–28
Pullarkat V, Blanchard S, Tegtmeier B et al (2008) Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 42:799–805
Chotsampancharoen T, Gan K, Kasow KA et al (2009) Iron overload in survivors of childhood leukemia after allogeneic hematopoietic stem cell transplantation. Pediatr Transplant 13:348–352
Neufeld EJ (2006) Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood 107:3436–3441
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steegmann-Olmedillas, J.L. The role of iron in tumour cell proliferation. Clin Transl Oncol 13, 71–76 (2011). https://doi.org/10.1007/s12094-011-0621-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-011-0621-1