Abstract
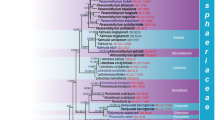
Phoma is a highly polyphyletic genus with its unclear species boundaries. The conventional system of identification is functional but it has its limitations. Besides morphological studies, chemotaxonomy, secondary metabolite and protein profiling have been assessed for the classification and identification of these fungi. Molecular datasets have provided a better outlook towards the phylogenetic and evolutionary trends of Phoma. Molecular markers such as ITS-rDNA, tubulin, actin, translation elongation factor have been widely used by the taxonomists to demarcate species. However, outcomes gained up till now represent preliminary step towards the study of Phoma systematics and a combined approach would be beneficial in the understanding of this polyphyletic group members. Lately, on the base of molecular phylogeny of the type species of the seven Phoma sections a new teleomorph family, Didymellaceae has been established, besides the Phaeosphaeriaceae related to sect. Paraphoma anamorphs, and the Leptosphaeriaceae to sect. Heterospora anamorphs. The estimated ratio is about 70 % of the recognized Phoma-like species can be associated with the Didymellaceae ascomycetous family.
Similar content being viewed by others
References
Aveskamp MM, de Gruyter J, Crous PW (2008) Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fung Diversity 31:1–18
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW (2010) Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Frisvad JC, Andersen B, Thrane U (2008) The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol Res 112:231–240
Saccardo PA (1880) Conspectus generum fungorum Italiae inferiorum, nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium, systemate sporologico dispositorum. Michelia 2:1–38
Boerema GH, Bollen GJ (1975) Conidiogenesis and conidial septation as differentiating criteria between Phoma and Ascochyta. Persoonia 8:111–144
Boerema GH (1997) Contributions towards a monograph of Phoma (Coelomycetes)—V. subdivision of the genus in sections. Mycotaxon 64:321–333
Boerema GH, de Gruyter J, Noordeloos ME, Hamers MEC (2004) Phoma identification manual: differentiation of specific and infra-specific taxa in culture. CABI Publishing, Oxfordshire
Kövics GJ, Sándor E, Rai MR, Irinyi L (2013) Phoma-like fungi of soybeans. Crit Rev Microbiol. http://informahealthcare.com/doi/abs/10.3109/1040841X.2012.755948
de Gruyter J, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 113:508–519
Rajak RC, Rai MK (1983) Effect of different factors on the morphology and cultural characters of 18-species and 5-varieties of Phoma. I. Effect of different media. Bibl Mycologia 91:301–317
Rajak RC, Rai MK (1984) A new leaf-spot disease of Jasminum pubescence caused by Phoma herbarum. Indian J Mycol Plant Pathol 42(2):173
Irinyi L, Kövics GJ, Rai MK, Sándor E (2006) Studies of evolutionary relationships of Phoma species based on phylogenetic markers. pp. 99–113 In: 4th International Plant Protection Symposium at Debrecen University, 18–19 October 2006. Recent developments of IPM. Proceedings. Kövics GJ, Dávid I (eds) Debrecen University, Hungary
Heiny DK (1990) Phoma probocis sp. nov. pathogenic on Convolvulus arvensis. Mycotaxon 36:457–471
Heiny DK (1994) Field survival of Phoma proboscis and synergism with herbicides for control of field bindweed. Plant Dis 78:1156–1164
Rajak RC, Farkya S, Hasija SK, Pandey AK (1990) Fungi associated with congress weed (Parthenium hysterophorus L.). Proc Nat Acad Sci India 60:165–168
Heiny DK, Templeton GE (1991) Effects of spore concentration, temperature and dew period on disease of field bindweed caused by Phoma proboscis. Phytopathology 81:905–909
Fogliano V, Marchese A, Scaloni A, Ritieni A, Visconti AG, Randazzo G, Graniti A (1998) Characterization of a 60 kDa phytotoxic glycoprotein produced by Phoma tracheiphila and its relation to malseccin. Physiol Mol Plant Pathol 53(3):149–161
Baxter CJ, Magan N, Lane B, Wildman HG (1998) Influence of water activity and temperature on in vitro growth of surface cultures of a Phoma sp., and production of the pharmaceutical metabolites, squalestatin S1 and S2. Appl Microbiol Biotechnol 3:328–332
Pandey S, Pandey AK (2000) Mycoherbicidal potential of some fungi against Lantana camara L.: a preliminary observation. J Trop For 16:28–32
Rai MK (2002) Diversity and biotechnological applications of Indian species of Phoma. In: Rao GP, Manoharachari C, Bhat DJ, Rajak RC, Lakhanpal TN (eds) Frontiers of fungal diversity in India. International Book Distributing Co, Lucknow, pp 179–204
Zimin L, Paul RJ, William F (2003) A cyclic carbonate and related polyketides from a marine-derived fungus of the genus Phoma. Phytochemistry 64:571–574
Shibazaki M, Taniguchi M, Yokoi T, Nagai K, Watanabe M, Suzuki K, Yamamoto T (2004) YM-215343, a novel antifungal compound from Phoma sp. QNO4621. J Antibiot 57:379–382
Koyama N, Nagahiro T, Yamaguchi Y, Ohshiro T, Masuma R, Tomoda H, Omura S (2005) Spylidone, a novel inhibitor of lipid droplet accumulation in mouse macrophages produced by Phoma sp. FKI-1840. J Antibiot 58:338–345
Cimmino A, Andolfi A, Berestetskiy A, Evidente A (2008) Production of phytotoxins by Phoma exigua var. exigua, a potential mycoherbicide against perennial thistles. J Agric Food Chem 56:630–634
Pedras M, Soledade C, Yang Y (2008) Structural and biological activity of maculansin A, a phytotoxin from the phytopathogenic fungus Leptosphaeria maculans. Phytochemistry 69:2966–2971
Hoffman AM, Mayer SG, Strobel GA, Hess WM, Sovocool GW, Grange AH, Harper JK, Arif AM, Grant DM, Kelley-Swift EG (2008) Purification, identification and activity of phomodione, a furandione from an endophytic Phoma species. Phytochemistry 69:1049–1056
Liermann JC, Kolshorn H, Opatz T, Thines E, Anke H (2009) Xanthepinone, an antimicrobial polyketide from a soil fungus closely related to Phoma medicaginis. J Nat Prod 72:1905–1907
Rai MK, Deshmukh P, Gade A, Ingle A, Kövics GJ, Irinyi L (2009) Phoma Saccardo: distribution, secondary metabolite production and biotechnological applications. Crit Rev Microbiol 35:182–196
Qin S, Hussain H, Schulz B, Draeger S, Krohn K (2010) Two new metabolites, epoxydine A and B, from Phoma sp. Helv Chim Acta 93:169–174
Pellegrino C, Gilardi G, Gullino ML, Garibaldi A (2010) Detection of Phoma valerianellae in lamb’s lettuce seeds. Phytoparasitica 38:159–165
Garibaldi A, Gilardi G, Gullino ML (2010) First report of leaf spot caused by Phoma multirostrata on Fuchsia × hybrida in Italy. Plant Dis 94:382
Strobel G, Singh SK, Riyaz-Ul-Hassan S, Mitchell AM, Geary B, Sears J (2011) An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol Lett 320:87–94
Wang X, Wang J, Gao J, Yang L (2012) First report of leaf spot disease on Schisandra chinensis caused by Phoma glomerata in China. Plant Dis 96:289
Li YP, Wright DG, Lanoiselet V, Wang CP, Eyres N, Real D, You MP, Barbetti MJ (2012) First report of Phoma herbarum on tedera (Bituminaria bituminosa var. albomarginata) in Australia. Plant Dis 96:769
Patil VB, Mali AM, Mahamuni RJ, Chavan NS, Kamble SS (2012) First report of leaf spot caused by Phoma costarricensis on Delphinium malabaricum in Western Ghats of India. Plant Dis 96:1074
Rajak RC, Rai MK (1982) Effect of different colours of light and temperatures on the morphology of three species of Phoma in vitro. Jabalpur Univ 1:6
Rai MK (1993) Identity and taxonomy of hitherto unreported pathogen causing leaf-spot disease of ginger in India. Mycotaxon 46:329–333
Rai MK, Rajak RC (1993) Effect of different factors on the morphology and cultural characters of 18 species and 5 varieties of Phoma III. effect of different carbon sources. Indian J Mycol Plant Pathol 23:311–313
Boerema GH, de Gruyter J (1998) Contributions towards a monograph of Phoma (Coelomycetes)—VII. Section Sclerophomella: taxa with thick-walled pseudoparenchymatous pycnidia. Persoonia 17:81–95
Boerema GH, de Gruyter J (1999) Contributions towards a monograph of Phoma (Coelomycetes) III.—Supplement. Additional species of section Plenodomus. Persoonia 17:273–280
Kövics GJ, Pandey AK, Rai MK (2005) Phoma Saccardo and related genera: some new perspectives in taxonomy and biotechnology. In: Deshmukh SK, Rai MK (eds) Biodiversity of fungi: their role in human life. Science Publishers Inc, Enfield (NH), Plymouth, pp 129–154
Bakerspigel A, Lowe D, Rostras A (1981) The isolation of Phoma eupyrena from a human lesion. Arch Dermatol 117:362–363
Rai MK (1989) Phoma sorghina infection in human being. Mycopathol 105:167–170
Rosen T, Rinaldi MJ, Tschen JA, Stern JK, Cernoch P (1996) Cutaneous lesions due to Pleurophoma (Phoma) complex. Southern Med J 89:431–433
Rishi K, Font RL (2003) Keratitis caused by an unusual fungus. Phoma species. Cornea 22:166–168
Balis E, Velegraki A, Fragou A, Pefanis A, Kalabokas T, Mountokalakis T (2006) Lung mass caused by Phoma exigua. Scand J Infect Dis 38:552–555
Tullio V, Banche G, Allizond V, Roana J, Mandras N, Scalasa D, Panzoneb M, Cervetti O, Valle S, Carlone N, Cuffini AM (2010) Non-dermatophyte moulds as skin and nail foot mycosis agents: Phoma herbarum, Chaetomium globosum and Microascus cinereus. Fung Biol 114:345–349
Roehm CE, Salazar JC, Haqstrom N, Valdez TA (2012) Phoma and Acremonium invasive fungal rhinosinusitis in congenital acute lymphocytic leukemia and literature review. Int J Pediatr Otorhi 76:1387–1391
Irinyi L, Kövics GJ, Sándor E (2009) Taxonomical re-evalution of Phoma-like soybean pathogenic fungi. Mycol Res 113:249–260
Kövics GJ (1995) Comments to the taxonomical problems of some plant pathogenic fungi (genera Ascochyta, Phoma, Phyllosticta). Review. (in Hungarian with English summary) Növényvédelem (Plant Protection) 31:307–315
Kövics GJ, de Gruyter J, van der Aa HA (1999) Phoma sojicola comb. nov., and other hyaline-spored coelomycetes pathogenic on soybean. Mycol Res 103:1065–1070
Castell-Miller CV, Zeyen RJ (2007) Infection and development of Phoma medicaginis on moderately resistant and susceptible alfalfa genotypes. Can J Plant Pathol 29:290–298
Guarro J, Gene J, Stchigel AM (1999) Developments in fungal taxonomy. Clin Microbiol Rev 12:454–500
Tandon RN, Bilgrami KS (1960) A new species of Phoma on the phylloclades of Muehlenbeckia platyclados. Proc Nat Acad Sci India 30B:331–333
Bilgrami KS (1963) Association of a new species of Phoma with Pleospora herbarum (Pers.) Rabh. Curr Sci 32:174–175
Agarwal GP, Sahni VP (1964) Fungi causing plant diseases at Jabalpur (Madhya Pradesh)-IX. Mycopathol 22:245–248
Dutta BG, Ghosh GR (1965) Soil fungi from Orissa IV. soil fungi of paddy fields. Mycologia 25:316–322
Chandra S, Tandon RN (1965) Two new leaf-spot fungi. Curr Sci 34:565–566
Chandra S, Tandon RN (1966) Three new leaf-infecting fungi from Allahabad. Mycopathol 29:273–276
Hasija SK (1966) Additions to the fungi of Jabalpur (Madhya Pradesh) V. Mycopathol 28:33–41
Shreemali JL (1972) Some new members of Sphaeropsidales from India. Indian Phytopathol 25:58–60
Shreemali JL (1973) Some new leaf infecting fungi. Indian J Mycol Plant Pathol 3:112–116
Jamaluddin M, Tandon P, Tandon RN (1975) A fruit rot of aonla (Phyllanthus emblica L.) caused by Phoma sp. Proc Nat Acad Sci India 45:75–76
Rai JN, Misra JK (1981) A new species of Phoma from Indian alkaline soil. Curr Sci 50:377
Rao S, Thirumalachar U (1981) Phoma exigua infecting brinjal leaves. Indian Phytopath 34:37
Rai MK (1985) Taxonomic studies of species of Phoma isolated from air. J Econ Taxon Bot 7:645–647
Rai MK (1986) Two new diseases of Albizzia lebbek and Jasminum sambac. Acta Bot Indica 14:170–171
Rai MK (1986) Two new diseases of cultivated plant. Acta Bot Indica 14:238–239
Rai MK (2000) Phoma research in India: a review. In: Rai MK, Varma A, Rajak RC (eds) Integrated management of plant resources. Scientific Publisher (India), Jodhpur, pp 337–371
Rai MK, Rajak RC (1982) A new leaf-spot disease of Ailanthus excelsa Roxb. Curr Sci 51:98–99
Rai MK, Rajak RC (1982) A report of leaf-spot disease of Holoptelea integrifolia planch caused by Phoma sorghina. Indian J Mycol Plant Path 12:342
Rai MK, Rajak RC (1986/1987) A new disease of Citrus medica and the identity of its causal organism Phoma exigua var. foeveta (Foister) Boerema. Indian J Mycol Plant Pathol 16:320–321
Rai MK, Rajak RC (1993) Distinguishing characteristics of some Phoma species. Mycotaxon 48:389–414
Rajak RC, Rai MK (1982) Species of Phoma from legumes. Indian Phytopath 35:609–611
Rajak RC, Rai MK (1984) Effect of different factors on the morphology and cultural characters of 18 species and 5 varieties of Phoma. II. Effect of different pH. Nova Hedwigia 40:299–311
Aveskamp MM, Verkley GJM, de Gruyter J, Murace MA, Perello A, Woudenberg JHC, Groenewald JZ, Crous PW (2009) DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101:363–382
Noordeloos ME, de Gruyter J, Eijk GW, Roeijmans HJ (1993) Production of dendritic crystals in pure cultures of Phoma and Ascochyta and its value as a taxonomic character relative to morphology, pathology and cultural characteristics. Mycol Res 97:1343–1350
Kövics GJ, de Gruyter J (1995) Comparative studies of esterase isozyme patterns of some Phoma species occurring on soybean. (in Hungarian with English summary). Sci Public Agric Univ Debrecen 31:191–207
Tiwari VV, Gade AK, Rai MK (2013) A study of phylogenetic variations among Indian Phoma tropica species by RAPD-PCR and ITS-rDNA sequencing. Ind J Biotechnol 12:187–194
Irinyi L, Gade AK, Ingle AP, Kövics GJ, Rai MK, Sándor E (2009) Morphology and molecular biology of Phoma. In: Gherbawy Y, Mach RL, Rai MK (eds) Current advances in molecular mycology. Nova Science Publishers Inc, New York, pp 171–203
Aveskamp MM, Woudenberg JHC, de Gruyter J, Turco E, Groenewald JZ, Crous PW (2009) Development of taxon-specific sequence characterized amplified region (SCAR) markers based on actin sequences and DNA amplification fingerprinting (DAF): a case study in the Phoma exigua species complex. Mol Plant Pathol 10:403–414
de Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2010) Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102:1066–1081
Goker M, Garcia-Blazquez G, Voglmayr H, Telleria MT, Martin MP (2009) Molecular taxonomy of phytopathogenic fungi: a case study in Peronospora. PLoS ONE 4:e6319
Knowlton N (1993) Sibling species in the sea. Annu Rev Ecol Syst 24:189–216
Grube M, Kroken S (2000) Molecular approaches and concept of species and species complexes in lichenized fungi. Mycol Res 104:1284–1294
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl Env Microbiol 61:1323–1330
Cleveland DW, Sullivan KF (1985) Molecular biology and genetics of tubulin. Annu Rev Biochem 54:331–365
Joshi HC, Cleveland DW (1990) Diversity among tubulin subunits: toward what functional end? Cell Motil Cytoskel 16:159–163
Löwe J, Li H, Downing KH, Nogales E (2001) Refined structure of alpha beta-tubulin at 3.5 a resolution. J Mol Biol 313:1045–1057
Saussede-Aim J, Dumontet C (2009) Regulation of tubulin expression: multiple overlapping mechanisms. Int J Med Medic Sci 1:290–296
Lee RCH, Williams BAP, Brown AMV, Adamson ML, Keeling PJ (2008) α- and β-tubulin phylogenies support a close relationship between the microsporidia Brachiola algerae and Antonospora locustae. J Eukaryot Microbiol 55:388–392
Dangre DM, Rathod DP, Gade AK, Rai MK (2009) An in silico molecular evolutionary analysis of selected species of Phoma: a comparative approach. J Proteomics Bioinform 2:295–309
Hays SM, Swanson J, Selker EU (2002) Identification and characterization of the genes encoding the core histones and histone variants of Neurospora crassa. Genetics 160:961–973
Luger K (2006) Dynamic nucleosomes. Chromosome Res 14:5–16
Yun CS, Nishida H (2011) Distribution of introns in fungal histone genes. PLoS ONE 6:e16548
Wunsch MJ, Bergstrom GC (2011) Genetic and morphological evidence that Phoma sclerotioides, a causal agent of brown root rot of alfalfa, is composed of a species complex. Phytopathology 101:594–610
Goldstein PZ, DeSalle R (2010) Integrating DNA barcode data and taxonomic practice: determination, discovery and description. BioEssays 33:135–147
Seifert KA (2008) Integrating DNA barcoding into the mycological sciences. Persoonia 21:162–167
Seifert KA (2009) Progress towards DNA barcoding of fungi. Mol Ecol Res 9:83–89
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rai, M.K., Tiwari, V.V., Irinyi, L. et al. Advances in Taxonomy of Genus Phoma: Polyphyletic Nature and Role of Phenotypic Traits and Molecular Systematics. Indian J Microbiol 54, 123–128 (2014). https://doi.org/10.1007/s12088-013-0442-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-013-0442-8