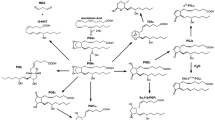
Abstract
Eicosanoid signaling controls a wide range of biological processes from blood pressure homeostasis to inflammation and resolution thereof to the perception of pain and to cell survival itself. Disruption of normal eicosanoid signaling is implicated in numerous disease states. Eicosanoid signaling is facilitated by G-protein-coupled, eicosanoid-specific receptors and the array of associated G-proteins. This review focuses on the expression, characterization, regulation, and mechanism of action of non-prostanoid, eicosanoid receptors.


Similar content being viewed by others
References
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341. https://doi.org/10.1124/pr.58.3.3
Adolfsson JL, Ohd JF, Sjölander A (1996) Leukotriene D4-induced activation and translocation of the G-protein alpha i3-subunit in human epithelial cells. Biochem Biophys Res Commun 226:413–419. https://doi.org/10.1006/bbrc.1996.1370
Aizawa F, Nishinaka T, Yamashita T, Nakamoto K, Kurihara T, Hirasawa A, Kasuya F, Miyata A, Tokuyama S (2016) GPR40/FFAR1 deficient mice increase noradrenaline levels in the brain and exhibit abnormal behavior. J Pharmacol Sci 132:249–254. https://doi.org/10.1016/j.jphs.2016.09.007
Andrew W, Martino B, Stefan B, Gabriel S, Gerardo T, Rafal GH, Florian T, de Beer TAP, Christine R, Lorenza B, Rosalba L, Torsten S (2018) SWISS-MODEL homology modelling of protein structures and complexes. Nucleic Acids Research 46(1):296–303
Aratake Y, Okuno T, Matsunobu T, Saeki K, Takayanagi R, Furuya S, Yokomizo T (2012) Helix 8 of leukotriene B4 receptor 1 inhibits ligand-induced internalization. FASEB J 26:4068–4078. https://doi.org/10.1096/fj.12-212050
Arcemisbéhère L, Sen T, Boudier L, Balestre MN, Gaibelet G, Detouillon E, Orcel H, Mendre C, Rahmeh R, Granier S, Vivès C, Fieschi F, Damian M, Durroux T, Banères JL, Mouillac B (2010) Leukotriene BLT2 receptor monomers activate the G(i2) GTP-binding protein more efficiently than dimers. J Biol Chem 285:6337–6347. https://doi.org/10.1074/jbc.M109.083477
Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN (2005) Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 201:713–722. https://doi.org/10.1084/jem.20042031
Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 178:3912–3917. https://doi.org/10.4049/jimmunol.178.6.3912
Austen KF, Maekawa A, Kanaoka Y, Boyce JA (2009) The leukotriene E4 puzzle: finding the missing pieces and revealing the pathobiologic implications. J Allergy Clin Immunol 124:406–414. https://doi.org/10.1016/j.jaci.2009.05.046
Bäck M, Bu DX, Bränström R, Sheikine Y, Yan ZQ, Hansson GK (2005) Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci U S A 102:17501–17506. https://doi.org/10.1073/pnas.0505845102
Bankova LG, Lai J, Yoshimoto E, Boyce JA, Austen KF, Kanaoka Y, Barrett NA (2016) Leukotriene E4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99. Proc Natl Acad Sci U S A 113:6242–6247. https://doi.org/10.1073/pnas.1605957113
Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Léon C, Gachet C (2005) Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol 67:721–733. https://doi.org/10.1124/mol.104.004846
Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y (2004) Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci U S A 101:3047–3052. https://doi.org/10.1073/pnas.0400235101
Benítez-Angeles M, Morales-Lázaro SL, Juárez-González E, Rosenbaum T (2020) TRPV1: structure, endogenous agonists, and mechanisms. Int J Mol Sci 21:3421. https://doi.org/10.3390/ijms21103421
Berg V, Sveinbjörnsson B, Bendiksen S, Brox J, Meknas K, Figenschau Y (2010) Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21–157). Arthritis Res Ther 12:R228. https://doi.org/10.1186/ar3215
Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW 4th (2002) cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35:721–731. https://doi.org/10.1016/s0896-6273(02)00802-4
Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW 4th (2003) Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc Natl Acad Sci U S A 100:12480–12485. https://doi.org/10.1073/pnas.2032100100
Binda C, Génier S, Cartier A, Larrivée JF, Stankova J, Young JC, Parent JL (2014) A G protein-coupled receptor and the intracellular synthase of its agonist functionally cooperate. J Cell Biol 204:377–393. https://doi.org/10.1083/jcb.201304015
Biringer RG (2018) The enzymes of the human eicosanoid pathway. Res Rep Med Sci 2:106. https://doi.org/10.6084/m9.figshare.11649351
Biringer RG (2020) A review of prostanoid receptors: expression, characterization, regulation, and mechanism of action. J Cell Commun Signal. https://doi.org/10.1007/s12079-020-00585-0
Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294:1351–1362. https://doi.org/10.1006/jmbi.1999.3310
Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S (2004) Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4:1633–1649. https://doi.org/10.1002/pmic.200300771
Bochnowicz S, Underwood DC (1995) Dose-dependent mediation of leukotriene D4-induced airway microvascular leakage and bronchoconstriction in the guinea pig. Prostaglandins Leukot Essent Fatty Acids 52:403–411. https://doi.org/10.1016/0952-3278(95)90069-1
Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK (2003) Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol 64:1210–1216. https://doi.org/10.1124/mol.64.5.1210
Boehmler AM, Drost A, Jaggy L, Seitz G, Wiesner T, Denzlinger C, Kanz L, Möhle R (2009) The CysLT1 ligand leukotriene D4 supports alpha4beta1- and alpha5beta1-mediated adhesion and proliferation of CD34+ hematopoietic progenitor cells. J Immunol 182:6789–6798. https://doi.org/10.4049/jimmunol.0801525
Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S, Van Gool F, Communi D, De Vuyst P, Desmecht D, Parmentier M (2011) ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog 7:e1002358. https://doi.org/10.1371/journal.ppat.1002358
Botten N, Hodges RR, Li D, Bair JA, Shatos MA, Utheim TP, Serhan CN, Dartt DA (2019) Resolvin D2 elevates cAMP to increase intracellular [Ca2+] and stimulate secretion from conjunctival goblet cells. FASEB J 33:8468–8478. https://doi.org/10.1096/fj.201802467R
Brash AR (1999) Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem 274:23679–23682. https://doi.org/10.1074/jbc.274.34.23679
Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR Jr, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI (2003) The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem 278:11303–11311. https://doi.org/10.1074/jbc.M211495200
Brochu-Bourque A, Véronneau S, Rola-Pleszczynski M, Stankova J (2011) Differential signaling defects associated with the M201V polymorphism in the cysteinyl leukotriene type 2 receptor. J Pharmacol Exp Ther 336:431–439. https://doi.org/10.1124/jpet.110.172411
Burnstock G (2018) Purine and purinergic receptors. Brain Neurosci Adv 2:2398212818817494. https://doi.org/10.1177/2398212818817494
Bye AP, Unsworth AJ, Gibbins JM (2016) Platelet signaling: a complex interplay between inhibitory and activatory networks. J Thromb Haemost 14:918–930. https://doi.org/10.1111/jth.13302
Capra V, Nicosia S, Ragnini D, Mezzetti M, Keppler D, Rovati GE (1998) Identification and characterization of two cysteinyl-leukotriene high affinity binding sites with receptor characteristics in human lung parenchyma. Mol Pharmacol 5:750–758. https://doi.org/10.1124/mol.53.4.750
Capra V, Accomazzo MR, Ravasi S, Parenti M, Macchia M, Nicosia S, Rovati GE (2003) Involvement of prenylated proteins in calcium signaling induced by LTD4 in differentiated U937 cells. Prostaglandins Other Lipid Mediat 71:235–251. https://doi.org/10.1016/s1098-8823(03)00045-5
Capra V, Ravasi S, Accomazzo MR, Citro S, Grimoldi M, Abbracchio MP, Rovati GE (2005) CysLT1 receptor is a target for extracellular nucleotide-induced heterologous desensitization: a possible feedback mechanism in inflammation. J Cell Sci 118(Pt 23):5625–5636. https://doi.org/10.1242/jcs.02668
Carnini C, Accomazzo MR, Borroni E, Vitellaro-Zuccarello L, Durand T, Folco G, Rovati GE, Capra V, Sala A (2011) Synthesis of cysteinyl leukotrienes in human endothelial cells: subcellular localization and autocrine signaling through the CysLT2 receptor. FASEB J 25:3519–3528. https://doi.org/10.1096/fj.10-177030
Caterina MJ, Julius D (2001) The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24:487–517. https://doi.org/10.1146/annurev.neuro.24.1.487
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Cattaneo F, Parisi M, Ammendola R (2013) Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci 14:7193–7230. https://doi.org/10.3390/ijms14047193
Chiang N, Fierro IM, Gronert K, Serhan CN (2000) Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med 191:1197–1208. https://doi.org/10.1084/jem.191.7.1197
Chiang N, Serhan CN, Dahlén SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C (2006) The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev 58:463–487. https://doi.org/10.1124/pr.58.3.4
Chiang N, Dalli J, Colas RA, Serhan CN (2015) Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 212:1203–1217. https://doi.org/10.1084/jem.20150225
Chiang N, de la Rosa X, Libreros S, Serhan CN (2017) Novel resolvin D2 receptor axis in infectious inflammation. J Immunol 198:842–851. https://doi.org/10.4049/jimmunol.1601650
Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP (2006) The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J 25:4615–4627. https://doi.org/10.1038/sj.emboj.7601341
Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME (2014) Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol 171:3908–3917. https://doi.org/10.1111/bph.12746
Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M (2013) Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A 110:18232–18237. https://doi.org/10.1073/pnas.1308253110
Cortright DN, Crandall M, Sanchez JF, Zou T, Krause JE, White G (2001) The tissue distribution and functional characterization of human VR1. Biochem Biophys Res Commun 281:1183–1189. https://doi.org/10.1006/bbrc.2001.4482
Cronin A, Decker M, Arand M (2011) Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase. J Lipid Res 52:712–719. https://doi.org/10.1194/jlr.M009639
Crooke ST, Mattern M, Sarau HM, Winkler JD, Balcarek J, Wong A, Bennett CF (1989) The signal transduction system of the leukotriene D4 receptor. Trends Pharmacol Sci 10:103–107. https://doi.org/10.1016/0165-6147(89)90206-x
Crooks SW, Stockley RA (1998) Leukotriene B4. Int J Biochem Cell Biol 30:173–178
Czech W, Barbisch M, Tenscher K, Schöpf E, Schröder JM, Norgauer J (1997) Chemotactic 5-oxo-eicosatetraenoic acids induce oxygen radical production, Ca2+-mobilization, and actin reorganization in human eosinophils via a pertussis toxin-sensitive G-protein. J Invest Dermatol 108:108–112. https://doi.org/10.1111/1523-1747.ep12285653
da Silva EZ, Jamur MC, Oliver C (2014) Mast cell function: a new vision of an old cell. J Histochem Cytochem 62:698–738. https://doi.org/10.1369/0022155414545334
Daniele S, Trincavelli ML, Gabelloni P, Lecca D, Rosa P, Abbracchio MP, Martini C (2011) Agonist-induced desensitization/resensitization of human G protein-coupled receptor 17: a functional cross-talk between purinergic and cysteinyl-leukotriene ligands. J Pharmacol Exp Ther 338:559–567. https://doi.org/10.1124/jpet.110.178715
Daniele S, Trincavelli ML, Fumagalli M, Zappelli E, Lecca D, Bonfanti E, Campiglia P, Abbracchio MP, Martini C (2014) Does GRK-β arrestin machinery work as a “switch on” for GPR17-mediated activation of intracellular signaling pathways? Cell Signal 26:1310–1325. https://doi.org/10.1016/j.cellsig.2014.02.016
Dartt DA, Hodges RR, Serhan CN (2019) Immunoresolvent resolvin D1 maintains the health of the ocular surface. Adv Exp Med Biol 1161:13–25. https://doi.org/10.1007/978-3-030-21735-8_3
De Henau O, Degroot GN, Imbault V, Robert V, De Poorter C, Mcheik S, Galés C, Parmentier M, Springael JY (2016) Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 11:e0164179. https://doi.org/10.1371/journal.pone.0164179
De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V (2000) Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett 483:52–56. https://doi.org/10.1016/s0014-5793(00)02082-2
de Poorter C, Baertsoen K, Lannoy V, Parmentier M, Springael JY (2013) Consequences of ChemR23 heteromerization with the chemokine receptors CXCR4 and CCR7. PLoS ONE 8:e58075. https://doi.org/10.1371/journal.pone.0058075
Dedoni S, Campbell LA, Harvey BK, Avdoshina V, Mocchetti I (2018) The orphan G-protein-coupled receptor 75 signaling is activated by the chemokine CCL5. J Neurochem 146:526–539. https://doi.org/10.1111/jnc.14463
Drzazga A, Kristinsson H, Sałaga M, Zatorski H, Koziołkiewicz M, Gendaszewska-Darmach E, Bergsten P (2018) Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol Cell Endocrinol 472:117–125. https://doi.org/10.1016/j.mce.2017.12.002
Dupré DJ, Le Gouill C, Gingras D, Rola-Pleszczynski M, Stanková J (2004) Inverse agonist activity of selected ligands of the cysteinyl-leukotriene receptor 1. J Pharmacol Exp Ther 309:102–108. https://doi.org/10.1124/jpet.103.059824
Edfalk S, Steneberg P, Edlund H (2008) Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57:2280–2287. https://doi.org/10.2337/db08-0307
Espinosa K, Bossé Y, Stankova J, Rola-Pleszczynski M (2003) CysLT1 receptor upregulation by TGF-beta and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J Allergy Clin Immunol 111:1032–1040. https://doi.org/10.1067/mai.2003.1451
Fan F, Roman RJ (2017) GPR75 identified as the first 20-HETE receptor: a chemokine receptor adopted by a new family. Circ Res 120:1696–1698. https://doi.org/10.1161/CIRCRESAHA.117.311022
Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW, Roman RJ (2016) Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (landmark Ed) 21:1427–1463
Filep JG (2013) Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc Natl Acad Sci USA 110:18033–18034. https://doi.org/10.1073/pnas.1317798110
Finlay DB, Joseph WR, Grimsey NL, Glass M (2016) GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. Peer J 4:e1835. https://doi.org/10.7717/peerj.1835
Fiore S, Maddox JF, Perez HD, Serhan CN (1994) Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med 180:253–260. https://doi.org/10.1084/jem.180.1.253
Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS (2001) Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107:1591–1598. https://doi.org/10.1172/JCI12242
Foster HR, Fuerst E, Lee TH, Cousins DJ, Woszczek G (2013) Characterisation of P2Y(12) receptor responsiveness to cysteinyl leukotrienes. PLoS ONE 8:e58305. https://doi.org/10.1371/journal.pone.0058305
Foster HR, Fuerst E, Branchett W, Lee TH, Cousins DJ, Woszczek G (2016) Leukotriene E4 is a full functional agonist for human cysteinyl leukotriene type 1 receptor-dependent gene expression. Sci Rep 6:20461. https://doi.org/10.1038/srep20461
Franke H, Parravicini C, Lecca D, Zanier ER, Heine C, Bremicker K, Fumagalli M, Rosa P, Longhi L, Stocchetti N, De Simoni MG, Weber M (2013) Abbracchio MP (2013) Changes of the GPR17 receptor, a new target for neurorepair, in neurons and glial cells in patients with traumatic brain injury. Purinergic Signal 9:451–462. https://doi.org/10.1007/s11302-013-9366-3
Fratangeli A, Parmigiani E, Fumagalli M, Lecca D, Benfante R, Passafaro M, Buffo A, Abbracchio MP, Rosa P (2013) The regulated expression, intracellular trafficking, and membrane recycling of the P2Y-like receptor GPR17 in Oli-neu oligodendroglial cells. J Biol Chem 288:5241–5256. https://doi.org/10.1074/jbc.M112.404996
Fretland DJ, Anglin CP, Bremer M, Isakson P, Widomski DL, Paulson SK, Docter SH, Djuric SW, Penning TD, Yu S, McKearn JP (1995) Antiinflammatory effects of second-generation leukotriene B4 receptor antagonist, SC-53228: impact upon leukotriene B4- and 12(R)-HETE-mediated events. Inflammation 19:193–205. https://doi.org/10.1007/bf01534461
Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML (2017) 20-HETE signals through g-protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circ Res 120:1776–1788. https://doi.org/10.1161/CIRCRESAHA.116.310525
Gaudreau R, Le Gouill C, Venne MH, Stankova J, Rola-Pleszczynski M (2002) Threonine 308 within a putative casein kinase 2 site of the cytoplasmic tail of leukotriene B(4) receptor (BLT1) is crucial for ligand-induced, G-protein-coupled receptor-specific kinase 6-mediated desensitization. J Biol Chem 277:31567–31576. https://doi.org/10.1074/jbc.M202723200
Ge Y, Zhang S, Wang J, Xia F, Wan JB, Lu J, Ye RD (2020) Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J 34(5):6920–6933. https://doi.org/10.1096/fj.201903206R
Gençoğlu H, Şahin K, Jones PM (2019) Determining the insulin secretion potential for certain specific G-protein coupled receptors in MIN6 pancreatic beta cells. Turk J Med Sci 49:403–411. https://doi.org/10.3906/sag-1712-147
Gerhard DS, Wagner L, Feingold EA, Shenmen CM, Grouse LH, Schuler G et al (2004) The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC). Genome Res 14:2121–2127. https://doi.org/10.1101/gr.2596504
Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL (1998) Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest 101:1860–1869. https://doi.org/10.1172/JCI1339
Goldsmith ZG, Dhanasekaran DN (2007) G protein regulation of MAPK networks. Oncogene 26:3122–3142. https://doi.org/10.1038/sj.onc.1210407
Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH (2003) Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol 4:965–973. https://doi.org/10.1038/ni972
Gregus AM, Doolen S, Dumlao DS, Buczynski MW, Takasusuki T, Fitzsimmons BL, Hua XY, Taylor BK, Dennis EA, Yaksh TL (2012) Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc Natl Acad Sci USA 109:6721–6726. https://doi.org/10.1073/pnas.1110460109
Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H, Wu LJ (2016) Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun 55:82–92. https://doi.org/10.1016/j.bbi.2015.11.007
Guerrero-Alba R, Barragán-Iglesias P, González-Hernández A, Valdez-Moráles EE, Granados-Soto V, Condés-Lara M, Rodríguez MG, Marichal-Cancino BA (2019) Some prospective alternatives for treating pain: the endocannabinoid system and its putative receptors GPR18 and GPR55. Front Pharmacol 9:1496. https://doi.org/10.3389/fphar.2018.01496
Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A (2000) Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1). J Physiol 525:747–759. https://doi.org/10.1111/j.1469-7793.2000.t01-1-00747.x
Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee MJ, Honn KV (2011) Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem 286:33832–33840. https://doi.org/10.1074/jbc.M110.216564
Haeggström JZ (2000) Structure, function, and regulation of Leukotriene A4 Hydrolase. Am J Respir Crit Care Med 161:S25–S31. https://doi.org/10.1164/ajrccm.161.supplement_1.ltta-6
Harder DR, Campbell WB, Roman RJ (1995) Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 32:79–92. https://doi.org/10.1159/000159080
Hardy AR, Conley PB, Luo J, Benovic JL, Poole AW, Mundell SJ (2005) P2Y1 and P2Y12 receptors for ADP desensitize by distinct kinase-dependent mechanisms. Blood 105:3552–3560. https://doi.org/10.1182/blood-2004-07-2893
Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJ, Medhurst AD, Smith GD, Topp S, Murdock P, Sanger GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB (2000) Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain 88:205–215. https://doi.org/10.1016/s0304-3959(00)00353-5
He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L (2004) Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429:188–193. https://doi.org/10.1038/nature02488
Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O’Neill GP, Metters KM, Lynch KR, Evans JF (2000) Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem 275:30531–30536. https://doi.org/10.1074/jbc.M003490200
Hennen S, Wang H, Peters L, Merten N, Simon K, Spinrath A, Blättermann S, Akkari R, Schrage R, Schröder R, Schulz D, Vermeiren C, Zimmermann K, Kehraus S, Drewke C, Pfeifer A, König GM, Mohr K, Gillard M, Müller CE, Lu QR, Gomeza J, Kostenis E (2013) Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci Signal. https://doi.org/10.1126/scisignal.2004350
Herová M, Schmid M, Gemperle C, Hersberger M (2015) ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol 194:2330–2337. https://doi.org/10.4049/jimmunol.1402166
Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, Dartt DA (2017) Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol 10:46–57. https://doi.org/10.1038/mi.2016.33
Honn KV (2008) Characterization of a Novel 12(S)-HETE receptor and its role in prostate cancer progression. annual research report for U.S. army medical research and materiel command Fort Detrick, Maryland. Available from https://apps.dtic.mil/dtic/tr/fulltext/u2/a482663.pdf
Honn KV, Guo Y, Cai Y, Lee MJ, Dyson G, Zhang W, Tucker SC (2016) 12-HETER1/GPR31, a high-affinity 12(S)-hydroxyeicosatetraenoic acid receptor, is significantly up-regulated in prostate cancer and plays a critical role in prostate cancer progression. FASEB J 30:2360–2369. https://doi.org/10.1096/fj.201500076
Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, Suto N, Tsunoda S, Taniguchi T, Ohnuki T (2002) Identification of a novel human eicosanoid receptor coupled to G(i/o). J Biol Chem 277:31459–31465. https://doi.org/10.1074/jbc.M203194200
Hosoi T, Sugikawa E, Chikada A, Koguchi Y, Ohnuki T (2005) TG1019/OXE, a Galpha(i/o)-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis. Biochem Biophys Res Commun 334:987–995. https://doi.org/10.1016/j.bbrc.2005.06.191
Houthuijzen JM (2016) For better or worse: FFAR1 and FFAR4 signaling in cancer and diabetes. Mol Pharmacol 90:738–743. https://doi.org/10.1124/mol.116.105932
Huet E, Boulay F, Barral S, Rabiet MJ (2007) The role of beta-arrestins in the formyl peptide receptor-like 1 internalization and signaling. Cell Signal 19:1939–1948. https://doi.org/10.1016/j.cellsig.2007.05.006
Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, Funk CD (2004) Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation 110:3360–3366. https://doi.org/10.1161/01.CIR.0000147775.50954.AA
Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U (2000) Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97:6155–6160. https://doi.org/10.1073/pnas.97.11.6155
Ignatov A, Robert J, Gregory-Evans C, Schaller HC (2006) RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75. Br J Pharmacol 149:490–497. https://doi.org/10.1038/sj.bjp.0706909
Inbe H, Watanabe S, Miyawaki M, Tanabe E, Encinas JA (2004) Identification and characterization of a cell-surface receptor, P2Y15, for AMP and adenosine. J Biol Chem 279:19790–19799. https://doi.org/10.1074/jbc.M400360200
Ishii Y, Saeki K, Liu M, Sasaki F, Koga T, Kitajima K, Meno C, Okuno T, Yokomizo T (2016) Leukotriene B4 receptor type 2 (BLT2) enhances skin barrier function by regulating tight junction proteins. FASEB J 30:933–947. https://doi.org/10.1096/fj.15-279653
Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M (2003) Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 422:173–176. https://doi.org/10.1038/nature01478
Iyinikkel JR (2018) Identifying novel G protein-coupled receptor targets in pulmonary arterial hypertension: uncovering the role of GPR75. Dissertation, University of Aberdeen,UK. Available from: https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.774022
Jahnel R, Dreger M, Gillen C, Bender O, Kurreck J, Hucho F (2001) Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur J Biochem 268:5489–5496. https://doi.org/10.1046/j.1432-1033.2001.02500.x
Jala VR, Shao WH, Haribabu B (2005) Phosphorylation-independent beta-arrestin translocation and internalization of leukotriene B4 receptors. J Biol Chem 280:4880–4887. https://doi.org/10.1074/jbc.M409821200
Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA (2007) CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood 110:3263–3270. https://doi.org/10.1182/blood-2007-07-100453
Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, Turner J, Kettle R, Bouhelal R, Charlton S, Nirmala NR, Jarai G, Finan P (2003) Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils. Mol Pharmacol 63:471–477. https://doi.org/10.1124/mol.63.3.471
Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U (2004) Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem 279:7048–7054. https://doi.org/10.1074/jbc.M311448200
Kain V, Liu F, Kozlovskaya V, Ingle KA, Bolisetty S, Agarwal A, Khedkar S, Prabhu SD, Kharlampieva E, Halade GV (2017) Resolution agonist 15-epi-Lipoxin A4 programs early activation of resolving phase in post-myocardial infarction healing. Sci Rep 7:9999. https://doi.org/10.1038/s41598-017-10441-8
Kalyvianaki K, Gebhart V, Peroulis N, Panagiotopoulou C, Kiagiadaki F, Pediaditakis I, Aivaliotis M, Moustou E, Tzardi M, Notas G, Castanas E, Kampa M (2017) Antagonizing effects of membrane-acting androgens on the eicosanoid receptor OXER1 in prostate cancer. Sci Rep 7:44418. https://doi.org/10.1038/srep44418
Kalyvianaki K, Panagiotopoulos AA, Malamos P, Moustou E, Tzardi M, Stathopoulos EN, Ioannidis GS, Marias K, Notas G, Theodoropoulos PA, Castanas E, Kampa M (2019) Membrane androgen receptors (OXER1, GPRC6A AND ZIP9) in prostate and breast cancer: a comparative study of their expression. Steroids 142:100–108. https://doi.org/10.1016/j.steroids.2019.01.006
Kanaoka Y, Boyce JA (2014) Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res 6:288–295. https://doi.org/10.4168/aair.2014.6.4.288
Kanaoka Y, Maekawa A, Austen KF (2013) Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J Biol Chem 288:10967–10972. https://doi.org/10.1074/jbc.C113.453704
Kaneko K, Miyabe Y, Takayasu A, Fukuda S, Miyabe C, Ebisawa M, Yokoyama W, Watanabe K, Imai T, Muramoto K, Terashima Y, Sugihara T, Matsushima K, Miyasaka N, Nanki T (2011) Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ther 13:R158. https://doi.org/10.1186/ar3475
Kaplamadzhiev DB, Hisha H, Adachi Y, Ikehara S, Tonchev AB, Boneva NB, Pyko IV, Kikuchi M, Nakaya M, Wakayama T, Iseki S, Yamashima T (2010) Bone marrow-derived stromal cells can express neuronal markers by DHA/GPR40 signaling. Biosci Trends 4:119–129
Kauffenstein G, Hechler B, Cazenave JP, Gachet C (2004) Adenine triphosphate nucleotides are antagonists at the P2Y receptor. J Thromb Haemost 2:1980–1988. https://doi.org/10.1111/j.1538-7836.2004.00926.x
Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM (2001) Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem 276:28613–28619. https://doi.org/10.1074/jbc.M103272200
Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, Dubova I, Zaheer S, Iyer SS, Zaheer A (2019) Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer’s Disease. Front Cell Neurosci 13:54. https://doi.org/10.3389/fncel.2019.00054
Kim H, Choi JA, Park GS, Kim JH (2012) BLT2 up-regulates interleukin-8 production and promotes the invasiveness of breast cancer cells. PLoS ONE 7:e49186. https://doi.org/10.1371/journal.pone.0049186
Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K (2008) P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci 28:2892–2902. https://doi.org/10.1523/JNEUROSCI.5589-07.2008
Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M (2006) Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun 347:827–832. https://doi.org/10.1016/j.bbrc.2006.06.175
Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B (2003) A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun 301:406–410. https://doi.org/10.1016/s0006-291x(02)03064-4
Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A 107:1660–1665. https://doi.org/10.1073/pnas.0907342107
Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol 180:2018–2027. https://doi.org/10.1016/j.ajpath.2012.01.028
Kubala SA, Patil SU, Shreffler WG, Hurley BP (2014) Pathogen induced chemo-attractant hepoxilin A3 drives neutrophils, but not eosinophils across epithelial barriers. Prostaglandins Other Lipid Mediat 108:1–8. https://doi.org/10.1016/j.prostaglandins.2013.11.001
Kuniyeda K, Okuno T, Terawaki K, Miyano M, Yokomizo T, Shimizu T (2007) Identification of the intracellular region of the leukotriene B4 receptor type 1 that is specifically involved in Gi activation. J Biol Chem 282:3998–4006. https://doi.org/10.1074/jbc.M610540200
Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH (1993) Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet 341:989–990. https://doi.org/10.1016/0140-6736(93)91073-u
Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN (2013) Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498:371–375. https://doi.org/10.1038/nature12175
Landrum MJ, Lee JM, Benson M, Brown G, Chao C, Chitipiralla S, Gu B, Hart J, Hoffman D, Hoover J, Jang W, Katz K, Ovetsky M, Riley G, Sethi A, Tully R, Villamarin-Salomon R, Rubinstein W, Maglott DR (2016) ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res. https://doi.org/10.1093/nar/gkv1222
Langlois A, Ferland C, Tremblay GM, Laviolette M (2006) Montelukast regulates eosinophil protease activity through a leukotriene-independent mechanism. J Allergy Clin Immunol 118:113–119. https://doi.org/10.1016/j.jaci.2006.03.010
Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, Saijo K, Glass CK, Hume DA, Kellie S, Sweet MJ (2008) Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res 4:5. https://doi.org/10.1186/1745-7580-4-5
Le Y, Murphy PM, Wang JM (2002) Formyl-peptide receptors revisited. Trends Immunol 23:541–548. https://doi.org/10.1016/s1471-4906(02)02316-5
Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, Villa G, Verderio C, Grumelli C, Guerrini U, Tremoli E, Rosa P, Cuboni S, Martini C, Buffo A, Cimino M, Abbracchio MP (2008) The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE 3:e3579. https://doi.org/10.1371/journal.pone.0003579
Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, Serhan CN, Dartt DA (2013) Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol 6:1119–1130. https://doi.org/10.1038/mi.2013.7
Lindsay MA, Haddad EB, Rousell J, Teixeira MM, Hellewell PG, Barnes PJ, Giembycz MA (1998) Role of the mitogen-activated protein kinases and tyrosine kinases during leukotriene B4-induced eosinophil activation. J Leukoc Biol 64:555–562. https://doi.org/10.1016/s1471-4906(02)02316-5
Lippestad M, Hodges RR, Utheim TP, Serhan CN, Dartt DA (2017) Resolvin D1 increases mucin secretion in cultured rat conjunctival goblet cells via multiple signaling pathways. Invest Ophthalmol vis Sci 58:4530–4544. https://doi.org/10.1167/iovs.17-21914
Liu B, Khan WA, Hannun YA, Timar J, Taylor JD, Lundy S, Butovich I, Honn KV (1995) 12(S)-hydroxyeicosatetraenoic acid and 13(S)-hydroxyoctadecadienoic acid regulation of protein kinase C-alpha in melanoma cells: role of receptor-mediated hydrolysis of inositol phospholipids. Proc Natl Acad Sci U S A 92:9323–9327. https://doi.org/10.1073/pnas.92.20.9323
Liu M, Saeki K, Matsunobu T, Okuno T, Koga T, Sugimoto Y, Yokoyama C, Nakamizo S, Kabashima K, Narumiya S, Shimizu T, Yokomizo T (2014) 12-Hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J Exp Med 211(6):1063–1078. https://doi.org/10.1084/jem.20132063
Liu M, Shen J, Yuan H, Chen F, Song H, Qin H, Li Y, Xu J, Ye Q, Li S, Saeki K, Yokomizo T (2018) Leukotriene B4 receptor 2 regulates the proliferation, migration, and barrier integrity of bronchial epithelial cells. J Cell Physiol 233:6117–6124. https://doi.org/10.1002/jcp.26455
Lu G, Henderson D, Liu L, Reinhart PH, Simon SA (2005) TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol 67:1119–1127. https://doi.org/10.1124/mol.104.009852
Lu C, Dong L, Zhou H, Li Q, Huang G, Bai SJ, Liao L (2018) G-Protein-coupled receptor Gpr17 regulates oligodendrocyte differentiation in response to lysolecithin-induced demyelination. Sci Rep 8:4502. https://doi.org/10.1038/s41598-018-22452-0
Lundeen KA, Sun B, Karlsson L, Fourie AM (2006) Leukotriene B4 receptors BLT1 and BLT2: expression and function in human and murine mast cells. J Immunol 177:3439–3447. https://doi.org/10.4049/jimmunol.177.5.3439
Luo J, Swaminath G, Brown SP, Zhang J, Guo Q, Chen M, Nguyen K, Tran T, Miao L, Dransfield PJ, Vimolratana M, Houze JB, Wong S, Toteva M, Shan B, Li F, Zhuang R, Lin DC (2012) A potent class of GPR40 full agonists engages the enteroinsular axis to promote glucose control in rodents. PLoS ONE 7:e46300. https://doi.org/10.1371/journal.pone.0046300
Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL Jr, Ford-Hutchinson AW, Caskey CT, Evans JF (1999) Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 399:789–793. https://doi.org/10.1038/21658
Maddox JF, Serhan CN (1996) Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med 183:137–146. https://doi.org/10.1084/jem.183.1.137
Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN (1997) Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem 272:6972–6978. https://doi.org/10.1074/jbc.272.11.6972
Maddox JF, Colgan SP, Clish CB, Petasis NA, Fokin VV, Serhan CN (1998) Lipoxin B4 regulates human monocyte/neutrophil adherence and motility: design of stable lipoxin B4 analogs with increased biologic activity. FASEB J 12:487–494. https://doi.org/10.1096/fasebj.12.6.487
Maderna P, Godson C (2003) Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim Biophys Acta 1639:141–151. https://doi.org/10.1016/j.bbadis.2003.09.004
Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, Godson C (2010) FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J 24:4240–4249. https://doi.org/10.1096/fj.10-159913
Maekawa A, Austen KF, Kanaoka Y (2002) Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem 277:20820–20824. https://doi.org/10.1074/jbc.M203163200
Maekawa A, Kanaoka Y, Xing W, Austen KF (2008) Functional recognition of a distinct receptor preferential for leukotriene E4 in mice lacking the cysteinyl leukotriene 1 and 2 receptors. Proc Natl Acad Sci U S A 105:16695–16700. https://doi.org/10.1073/pnas.0808993105
Maekawa A, Balestrieri B, Austen KF, Kanaoka Y (2009) GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci USA 106:11685–11690. https://doi.org/10.1073/pnas.0905364106
Malfacini D, Patt J, Annala S, Harpsøe K, Eryilmaz F, Reher R, Crüsemann M, Hanke W, Zhang H, Tietze D, Gloriam DE, Bräuner-Osborne H, Strømgaard K, König GM, Inoue A, Gomeza J, Kostenis E (2019) Rational design of a heterotrimeric G protein α subunit with artificial inhibitor sensitivity. J Biol Chem 294:5747–5758. https://doi.org/10.1074/jbc.RA118.007250
Mancini AD, Bertrand G, Vivot K, Carpentier É, Tremblay C, Ghislain J, Bouvier M, Poitout V (2015) β-Arrestin recruitment and biased agonism at free fatty acid receptor 1. J Biol Chem 290:21131–21140. https://doi.org/10.1074/jbc.M115.644450
Mandadi S, Roufogalis BD (2008) ThermoTRP channels in nociceptors: taking a lead from capsaicin receptor TRPV1. Curr Neuropharmacol 6:21–38. https://doi.org/10.2174/157015908783769680
Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D (1987) The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience 23:275–289. https://doi.org/10.1016/0306-4522(87)90289-2
Marucci G, Dal Ben D, Lambertucci C, Santinelli C, Spinaci A, Thomas A, Volpini R, Buccioni M (2016) The G Protein-Coupled receptor GPR17: overview and update. ChemMedChem 11:2567–2574. https://doi.org/10.1002/cmdc.201600453
Mashiko M, Kurosawa A, Tani Y, Tsuji T, Takeda S (2019) GPR31 and GPR151 are activated under acidic conditions. J Biochem 166:317–322. https://doi.org/10.1093/jb/mvz042
Mathis SP, Jala VR, Lee DM, Haribabu B (2010) Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J Immunol 185:3049–3056. https://doi.org/10.4049/jimmunol.1001031
McCauley LK, Dalli J, Koh AJ, Chiang N, Serhan CN (2014) Cutting edge: parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. J Immunol 193:26–29. https://doi.org/10.4049/jimmunol.1301945
McHugh D, Page J, Dunn E, Bradshaw HB (2012) Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol 165:2414–2424. https://doi.org/10.1111/j.1476-5381.2011.01497.x
McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, Toms C, Peacock M, Shah K, Winter J, Weerasakera N, Webb M, Rang HP, Bevan S, James IF (2001) Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br J Pharmacol 132:1084–1094. https://doi.org/10.1038/sj.bjp.0703918
Mechiche H, Candenas L, Pinto FM, Nazeyrollas P, Clément C, Devillier P (2004) Characterization of cysteinyl leukotriene receptors on human saphenous veins: antagonist activity of montelukast and its metabolites. J Cardiovasc Pharmacol 43:113–120. https://doi.org/10.1097/00005344-200401000-00017
Mehdawi LM, Satapathy SR, Gustafsson A, Lundholm K, Alvarado-Kristensson M, Sjölander A (2017) A potential anti-tumor effect of leukotriene C4 through the induction of 15-hydroxyprostaglandin dehydrogenase expression in colon cancer cells. Oncotarget. https://doi.org/10.18632/oncotarget.16591
Melkes B, Markova V, Hejnova L, Marek A, Novotny J (2020) Naloxone is a potential binding ligand and activator of the capsaicin receptor TRPV1. Biol Pharm Bull 43:908–912. https://doi.org/10.1248/bpb.b19-00806
Mellor EA, Frank N, Soler D, Hodge MR, Lora JM, Austen KF, Boyce JA (2003) Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: functional distinction from CysLT1R. Proc Natl Acad Sci U S A 100:11589–11593. https://doi.org/10.1073/pnas.2034927100
Mogilner A, Oster G (1996) Cell motility driven by actin polymerization. Biophys J 71:3030–3045. https://doi.org/10.1016/S0006-3495(96)79496-1
Mohapatra DP, Nau C (2005) Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 280:13424–13432. https://doi.org/10.1074/jbc.M410917200
Montell C (2005) The TRP superfamily of cation channels. Sci STKE. https://doi.org/10.1126/stke.2722005re3
Moore AR, Ceraudo E, Sher JJ, Guan Y, Shoushtari AN, Chang MT, Zhang JQ, Walczak EG, Kazmi MA, Taylor BS, Huber T, Chi P, Sakmar TP, Chen Y (2016) Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat Genet 48:675–680. https://doi.org/10.1038/ng.3549
Mundell SJ, Jones ML, Hardy AR, Barton JF, Beaucourt SM, Conley PB, Poole AW (2006) Distinct roles for protein kinase C isoforms in regulating platelet purinergic receptor function. Mol Pharmacol 70:1132–1142. https://doi.org/10.1124/mol.106.023549
Munkarah A, Mert I, Chhina J, Hamid S, Poisson L, Hensley-Alford S, Giri S, Rattan R (2016) Targeting of free fatty acid receptor 1 in EOC: A novel strategy to restrict the adipocyte-EOC dependence. Gynecol Oncol 141:72–79. https://doi.org/10.1016/j.ygyno.2016.02.02
Naik S, Billington CK, Pascual RM, Deshpande DA, Stefano FP, Kohout TA, Eckman DM, Benovic JL, Penn RB (2005) Regulation of cysteinyl leukotriene type 1 receptor internalization and signaling. J Biol Chem 280:8722–8732. https://doi.org/10.1074/jbc.M413014200
Nakanishi M, Rosenberg DW (2013) Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol 35:123–137. https://doi.org/10.1007/s00281-012-0342-8
Nakanishi Y, Tan M, Ichiki T, Inoue A, Yoshihara JI, Maekawa N, Takenoshita I, Yanagida K, Yamahira S, Yamaguchi S, Aoki J, Nagamune T, Yokomizo T, Shimizu T, Nakamura M (2018) Stepwise phosphorylation of leukotriene B4 receptor 1 defines cellular responses to leukotriene B4. Sci Signal. https://doi.org/10.1126/scisignal.aao5390
Natarajan R, Nadler JL (2004) Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol 24:1542–1548. https://doi.org/10.1161/01.ATV.0000133606.69732.4c
Neves JS, Radke AL, Weller PF (2010) Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J Allergy Clin Immunol 125:477–482. https://doi.org/10.1016/j.jaci.2009.11.029
Nguyen CH, Stadler S, Brenner S, Huttary N, Krieger S, Jäger W, Dolznig H, Krupitza G (2016) Cancer cell-derived 12(S)-HETE signals via 12-HETE receptor, RHO, ROCK and MLC2 to induce lymph endothelial barrier breaching. Br J Cancer 115:364–370. https://doi.org/10.1038/bjc.2016.201
Nisar SP, Cunningham M, Saxena K, Pope RJ, Kelly E, Mundell SJ (2012) Arrestin scaffolds NHERF1 to the P2Y12 receptor to regulate receptor internalization. J Biol Chem 287:24505–24515. https://doi.org/10.1074/jbc.M112.347104
Nishi H, Pelleg A, Schulman ES (2016) IgE receptor-mediated histamine release in human lung mast cells: modulation by purinergic receptor ligands. Ann Clin Lab Sci 46:463–469
Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M (2006) Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 55(Suppl 2):S16–S23. https://doi.org/10.2337/db06-s003
Nonaka Y, Hiramoto T, Fujita N (2005) Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun 337:281–288. https://doi.org/10.1016/j.bbrc.2005.09.052
Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M (2012) Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol 32:1970–1978. https://doi.org/10.1161/ATVBAHA.112.249508
Nothacker HP, Wang Z, Zhu Y, Reinscheid RK, Lin SH, Civelli O (2000) Molecular cloning and characterization of a second human cysteinyl leukotriene receptor: discovery of a subtype selective agonist. Mol Pharmacol 58:1601–1608. https://doi.org/10.1124/mol.58.6.1601
Numazaki M, Tominaga T, Toyooka H, Tominaga M (2002) Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 277:13375–13378. https://doi.org/10.1074/jbc.C200104200
O’Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, Daniel LW (1996a) 5-Oxo-eicosatetraenoate is a broadly active, eosinophil-selective stimulus for human granulocytes. J Immunol 157:336–342
O’Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, Daniel LW (1996b) 5-Oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway. J Biol Chem 271:17821–17828. https://doi.org/10.1074/jbc.271.30.17821
O’Flaherty JT, Taylor JS, Thomas MJ (1998) Receptors for the 5-oxo class of eicosanoids in neutrophils. J Biol Chem 273:32535–32541. https://doi.org/10.1074/jbc.273.49.32535
Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN (2011) Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest 121:569–581. https://doi.org/10.1172/JCI42545
Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, Serhan CN (2012) Resolvin E2 formation and impact in inflammation resolution. J Immunol 188:4527–4534. https://doi.org/10.4049/jimmunol.1103652
Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN (2010) Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 285:3451–3461. https://doi.org/10.1074/jbc.M109.044131
Ohshima N, Nagase H, Koshino T, Miyamasu M, Yamaguchi M, Hirai K, Yamamoto K, Fujisawa T, Nakagawa N, Kishikawa K, Morita Y (2002) A functional study on CysLT(1) receptors in human eosinophils. Int Arch Allergy Immunol 129:67–75. https://doi.org/10.1159/000065175
Okuno T, Yokomizo T (2018) Biological functions of 12(S)-hydroxyheptadecatrienoic acid as a ligand of leukotriene B4 receptor 2. Inflamm Regen 38:29. https://doi.org/10.1186/s41232-018-0087-4
Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, Shimizu T (2008) 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2. J Exp Med 205:759–766. https://doi.org/10.1084/jem.20072329
Okuno T, Ishitani T, Yokomizo T (2015) Biochemical characterization of three BLT receptors in zebrafish. PLoS ONE 10:e0117888. https://doi.org/10.1371/journal.pone.0117888
Pace-Asciak CR (2015) Pathophysiology of the hepoxilins. Biochim Biophys Acta 1851:383–396. https://doi.org/10.1016/j.bbalip.2014.09.007
Pace-Asciak CR, Reynaud D, Demin P, Aslam R, Sun A (2002) A new family of thromboxane receptor antagonists with secondary thromboxane synthase inhibition. J Pharmacol Exp Ther 301:618–624. https://doi.org/10.1124/jpet.301.2.618
Parhamifar L, Sime W, Yudina Y, Vilhardt F, Mörgelin M, Sjölander A (2010) Ligand-induced tyrosine phosphorylation of cysteinyl leukotriene receptor 1 triggers internalization and signaling in intestinal epithelial cells. PLoS ONE 5:e14439. https://doi.org/10.1371/journal.pone.0014439
Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, Ji RR (2011) Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci 31:18433–18438. https://doi.org/10.1523/JNEUROSCI.4192-11.2011
Park SK, Herrnreiter A, Pfister SL, Gauthier KM, Falck BA, Falck JR, Campbell WB (2018) GPR40 is a low-affinity epoxyeicosatrienoic acid receptor in vascular cells. J Biol Chem 293:10675–10691. https://doi.org/10.1074/jbc.RA117.001297
Park J, Jang JH, Kim JH (2019) Mediatory role of BLT2 in the proliferation of KRAS mutant colorectal cancer cells. Biochim Biophys Acta Mol Cell Res 1866:329–336. https://doi.org/10.1016/j.bbamcr.2018.12.006
Parker WAE, Storey RF (2021) Novel approaches to P2Y12 inhibition and aspirin dosing. Platelets 32:7–14. https://doi.org/10.1080/09537104.2020.1714574
Parmentier CN, Fuerst E, McDonald J, Bowen H, Lee TH, Pease JE, Woszczek G, Cousins DJ (2012) Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1. J Allergy Clin Immunol 129:1136–1142. https://doi.org/10.1016/j.jaci.2012.01.057
Parravicini C, Ranghino G, Abbracchio MP, Fantucci P (2008) GPR17: molecular modeling and dynamics studies of the 3-D structure and purinergic ligand binding features in comparison with P2Y receptors. BMC Bioinformatics 9:263. https://doi.org/10.1186/1471-2105-9-263
Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA (2009) Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med 206(11):2543–2555. https://doi.org/10.1084/jem.20091240
Pierdomenico AM, Recchiuti A, Simiele F, Codagnone M, Mari VC, Davì G, Romano M (2015) MicroRNA-181b regulates ALX/FPR2 receptor expression and proresolution signaling in human macrophages. J Biol Chem 290:3592–3600. https://doi.org/10.1074/jbc.M114.592352
Piomelli D, Volterra A, Dale N, Siegelbaum SA, Kandel ER, Schwartz JH, Belardetti F (1987) Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature 328:38–43. https://doi.org/10.1038/328038a0
Pollock K, Creba J (1990) Leukotriene D4 induced calcium changes in U937 cells may utilize mechanisms additional to inositol phosphate production that are pertussis toxin insensitive but are blocked by phorbol myristate acetate. Cell Signal 2:563–568. https://doi.org/10.1016/0898-6568(90)90078-o
Powell WS, Rokach J (2013) The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor. Prog Lipid Res 52:651–665. https://doi.org/10.1016/j.plipres.2013.09.001
Powell WS, Rokach J (2015) Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta 1851:340–355. https://doi.org/10.1016/j.bbalip.2014.10.008
Powell WS, Rokach J (2020) Targeting the OXE receptor as a potential novel therapy for asthma. Biochem Pharmacol. https://doi.org/10.1016/j.bcp.2020.113930
Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M (1993) Stimulation of human neutrophils by 5-oxo-6,8,11,14-eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor. J Biol Chem 268:9280–9286
Powell WS, Chung D, Gravel S (1995) 5-Oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of human eosinophil migration. J Immunol 154:4123–4132
Powell WS, Gravel S, Halwani F (1999) 5-oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of L-selectin shedding, surface expression of CD11b, actin polymerization, and calcium mobilization in human eosinophils. Am J Respir Cell Mol Biol 20:163–170. https://doi.org/10.1165/ajrcmb.20.1.3141
Premkumar LS, Ahern GP (2000) Induction of vanilloid receptor channel activity by protein kinase C. Nature 408:985–990. https://doi.org/10.1038/35050121
Pugliese AM, Trincavelli ML, Lecca D, Coppi E, Fumagalli M, Ferrario S, Failli P, Daniele S, Martini C, Pedata F, Abbracchio MP (2009) Functional characterization of two isoforms of the P2Y-like receptor GPR17: [35S]GTPgammaS binding and electrophysiological studies in 1321N1 cells. Am J Physiol Cell Physiol 297:C1028-1040. https://doi.org/10.1152/ajpcell.00658.2008
Qi AD, Harden TK, Nicholas RA (2013) Is GPR17 a P2Y/leukotriene receptor? examination of uracil nucleotides, nucleotide sugars, and cysteinyl leukotrienes as agonists of GPR17. J Pharmacol Exp Ther 347:38–46. https://doi.org/10.1124/jpet.113.207647
Qiao N, Reynaud D, Demin P, Halushka PV, Pace-Asciak CR (2003) The thromboxane receptor antagonist PBT-3, a hepoxilin stable analog, selectively antagonizes the TPalpha isoform in transfected COS-7 cells. J Pharmacol Exp Ther 307:1142–1147. https://doi.org/10.1124/jpet.103.056705
Qin Y, Verdegaal EM, Siderius M, Bebelman JP, Smit MJ, Leurs R, Willemze R, Tensen CP, Osanto S (2011) Quantitative expression profiling of G-protein-coupled receptors (GPCRs) in metastatic melanoma: the constitutively active orphan GPCR GPR18 as novel drug target. Pigment Cell Melanoma Res 24:207–218. https://doi.org/10.1111/j.1755-148X.2010.00781.x
Rajkumar P, Pluznick JL (2017) Unsung renal receptors: orphan G-protein-coupled receptors play essential roles in renal development and homeostasis. Acta Physiol (oxf) 220:189–200. https://doi.org/10.1111/apha.12813
Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M (2002) PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci 22:4740–4745
Rempel V, Volz N, Gläser F, Nieger M, Bräse S, Müller CE (2013) Antagonists for the orphan G-protein-coupled receptor GPR55 based on a coumarin scaffold. J Med Chem 56:4798–4810. https://doi.org/10.1021/jm4005175
Rempel V, Atzler K, Behrenswerth A, Karcz T, Schoeder C, Hinz S, Kaleta M, Thimm D, Kiec-Kononowicz K, Müller CE (2014) Bicyclic imidazole-4-one derivatives: a new class of antagonists for the orphan G protein-coupled receptors GPR18 and GPR55. Med Chem Commun 5:632–649
Reyes-Resina I, Navarro G, Aguinaga D, Canela EI, Schoeder CT, Załuski M, Kieć-Kononowicz K, Saura CA, Müller CE, Franco R (2018) Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relev Neurodegen Dis Biochem Pharmacol 157:169–179. https://doi.org/10.1016/j.bcp.2018.06.001
Roman RJ (2002) P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82:131–185. https://doi.org/10.1152/physrev.00021.2001
Romano M (2006) Lipid mediators: lipoxin and aspirin-triggered 15-epi-lipoxins. Inflamm Allergy Drug Targets 5:81–90. https://doi.org/10.2174/187152806776383152
Romano M (2010) Lipoxin and aspirin-triggered lipoxins. Sci World J 10:1048–1064. https://doi.org/10.1100/tsw.2010.113
Romano M, Maddox JF, Serhan CN (1996) Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. J Immunol 157:2149–2154
Rosenbaum T, Awaya M, Gordon SE (2002) Subunit modification and association in VR1 ion channels. BMC Neurosci 3:4. https://doi.org/10.1186/1471-2202-3-4
Sadik CD, Sezin T, Kim ND (2013) Leukotrienes orchestrating allergic skin inflammation. Exp Dermatol 22:705–709. https://doi.org/10.1111/exd.12239
Saeki K, Yokomizo T (2017) Identification, signaling, and functions of LTB4 receptors. Semin Immunol 33:30–36. https://doi.org/10.1016/j.smim.2017.07.010
Salimi M, Stöger L, Liu W, Go S, Pavord I, Klenerman P, Ogg G, Xue L (2017) Cysteinyl leukotriene E4 activates human group 2 innate lymphoid cells and enhances the effect of prostaglandin D2 and epithelial cytokines. J Allergy Clin Immunol 140:1090-1100.e11. https://doi.org/10.1016/j.jaci.2016.12.958
Samuelsson B (1983) Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science 220:568–575. https://doi.org/10.1126/science.6301011
Sarau HM, Ames RS, Chambers J, Ellis C, Elshourbagy N, Foley JJ, Schmidt DB, Muccitelli RM, Jenkins O, Murdock PR, Herrity NC, Halsey W, Sathe G, Muir AI, Nuthulaganti P, Dytko GM, Buckley PT, Wilson S, Bergsma DJ, Hay DW (1999) Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol Pharmacol 56:657–663. https://doi.org/10.1124/mol.56.3.657
Sasaki F, Yokomizo T (2019) The leukotriene receptors as therapeutic targets of inflammatory diseases. Int Immunol 31:607–615. https://doi.org/10.1093/intimm/dxz044
Sauer CG, White K, Stöhr H, Grimm T, Hutchinson A, Bernstein PS, Lewis RA, Simonelli F, Pauleikhoff D, Allikmets R, Weber BH (2001) Evaluation of the G protein coupled receptor-75 (GPR75) in age related macular degeneration. Br J Ophthalmol 85:969–975. https://doi.org/10.1136/bjo.85.8.969
Schmid M, Gemperle C, Rimann N, Hersberger M (2016) Resolvin D1 Polarizes Primary Human Macrophages toward a Proresolution Phenotype through GPR32. J Immunol 196:3429–3437. https://doi.org/10.4049/jimmunol.1501701
Schumacher MA, Eilers H (2010) TRPV1 splice variants: structure and function. Front Biosci (landmark Ed) 15:872–882. https://doi.org/10.2741/3651
Seo JM, Cho KJ, Kim EY, Choi MH, Chung BC, Kim JH (2011) Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Exp Mol Med 43:129–137. https://doi.org/10.3858/emm.2011.43.3.014
Seo JM, Park S, Kim JH (2012) Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. J Biol Chem 287:13840–13849. https://doi.org/10.1074/jbc.M111.317131
Serhan CN, Petasis NA (2011) Resolvins and protectins in inflammation resolution. Chem Rev 111:5922–5943. https://doi.org/10.1021/cr100396c
Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N (2011) Novel anti-inflammatory–pro-resolving mediators and their receptors. Curr Top Med Chem 11:629–647. https://doi.org/10.2174/1568026611109060629
Sharif NA, Williams GW, Davis TL (2000) Pharmacology and autoradiography of human DP prostanoid receptors using [(3)H]-BWA868C, a DP receptor-selective antagonist radioligand. Br J Pharmacol 131:1025–1038. https://doi.org/10.1038/sj.bjp.0703686
Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U (2002) Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci U S A 99:10150–10155. https://doi.org/10.1073/pnas.152002699
Shirasaki H, Seki N, Fujita M, Kikuchi M, Kanaizumi E, Watanabe K, Himi T (2007) Agonist- and T(H)2 cytokine-induced up-regulation of cysteinyl leukotriene receptor messenger RNA in human monocytes. Ann Allergy Asthma Immunol 99:340–347. https://doi.org/10.1016/S1081-1206(10)60550-9
Siangjong L, Goldman DH, Kriska T, Gauthier KM, Smyth EM, Puli N, Kumar G, Falck JR, Campbell WB (2017) Vascular hepoxilin and trioxilins mediate vasorelaxation through TP receptor inhibition in mouse arteries. Acta Physiol (oxf) 219:188–201. https://doi.org/10.1111/apha.12642
Simon K, Hennen S, Merten N, Blättermann S, Gillard M, Kostenis E, Gomeza J (2016) The orphan G protein-coupled receptor GPR17 negatively regulates oligodendrocyte differentiation via Gαi/o and Its downstream effector molecules. J Biol Chem 291:705–718. https://doi.org/10.1074/jbc.M115.683953
Simon K, Merten N, Schröder R, Hennen S, Preis P, Schmitt NK, Peters L, Schrage R, Vermeiren C, Gillard M, Mohr K, Gomeza J, Kostenis E (2017) The orphan receptor GPR17 is unresponsive to uracil nucleotides and cysteinyl leukotrienes. Mol Pharmacol 91:518–532. https://doi.org/10.1124/mol.116.107904
Soulet C, Sauzeau V, Plantavid M, Herbert JM, Pacaud P, Payrastre B, Savi P (2004) Gi-dependent and -independent mechanisms downstream of the P2Y12 ADP-receptor. J Thromb Haemost 2:135–146. https://doi.org/10.1111/j.1538-7836.2004.00556.x
Southern C, Cook JM, Neetoo-Isseljee Z, Taylor DL, Kettleborough CA, Merritt A, Bassoni DL, Raab WJ, Quinn E, Wehrman TS, Davenport AP, Brown AJ, Green A, Wigglesworth MJ, Rees S (2013) Screening β-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen 18:599–609. https://doi.org/10.1177/1087057113475480
Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461:1287–1291. https://doi.org/10.1038/nature08541
Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M (2004) Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol 13:129–139. https://doi.org/10.1111/j.0906-6705.2004.0178.x
Steinke JW, Negri J, Payne SC, Borish L (2014) Biological effects of leukotriene E4 on eosinophils. Prostaglandins Leukot Essent Fatty Acids 91:105–110. https://doi.org/10.1016/j.plefa.2014.02.006
Sultana C, Shen Y, Rattan V, Kalra VK (1996) Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte-like HL-60 cells is linked to protein kinase C activation. J Cell Physiol 167:477–487. https://doi.org/10.1002/(SICI)1097-4652(199606)167:3%3c477::AID-JCP12%3e3.0.CO;2-1
Sun YP, Tjonahen E, Keledjian R, Zhu M, Yang R, Recchiuti A, Pillai PS, Petasis NA, Serhan CN (2009) Anti-inflammatory and pro-resolving properties of benzo-lipoxin A(4) analogs. Prostaglandins Leukot Essent Fatty Acids 81:357–366. https://doi.org/10.1016/j.plefa.2009.09.004
Szallasi A, Blumberg PM (1999) Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev 51:159–212
Szekeres CK, Tang K, Trikha M, Honn KV (2000) Eicosanoid activation of extracellular signal-regulated kinase1/2 in human epidermoid carcinoma cells. J Biol Chem 275:38831–38841. https://doi.org/10.1074/jbc.M002673200
Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD (2003) Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol 4:982–990. https://doi.org/10.1038/ni970
Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN (1997) Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med 185:1693–1704. https://doi.org/10.1084/jem.185.9.1693
Talat N, Diaz-Cano S, Schulte KM (2011) Inflammatory diseases of the parathyroid gland. Histopathology 59:897–908. https://doi.org/10.1111/j.1365-2559.2011.04001.x
Tang DG, Chen YQ, Honn KV (1996) Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc Natl Acad Sci USA 93:5241–5246. https://doi.org/10.1073/pnas.93.11.5241
Thompson MD, Capra V, Clunes MT, Rovati GE, Stankova J, Maj MC, Duffy DL (2016) Cysteinyl leukotrienes pathway genes, atopic asthma and drug response: from population isolates to large genome-wide association studies. Front Pharmacol 7:299. https://doi.org/10.3389/fphar.2016.00299
Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN (2006) Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol 13:1193–1202. https://doi.org/10.1016/j.chembiol.2006.09.011
Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21:531–543. https://doi.org/10.1016/s0896-6273(00)80564-4
Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE (2005) LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun 335:949–956. https://doi.org/10.1016/j.bbrc.2005.07.166
Town MH, Casals-Stenzel J, Schillinger E (1983) Pharmacological and cardiovascular properties of a hydantoin derivative, BW 245 C, with high affinity and selectivity for PGD2 receptors. Prostaglandins 25:13–28. https://doi.org/10.1016/0090-6980(83)90131-4
Trauelsen M, Lückmann M, Frimurer TM, Schwartz TW (2018) The HETE is on FFAR1 and pancreatic islet cells. Cell Metab 27:273–275. https://doi.org/10.1016/j.cmet.2018.01.006
Tunaru S, Chennupati R, Nüsing RM, Offermanns S (2016) Arachidonic acid metabolite 19(S)-HETE induces vasorelaxation and platelet inhibition by activating prostacyclin (IP) receptor. PLoS One. https://doi.org/10.1371/journal.pone.01636333
Tunaru S, Bonnavion R, Brandenburger I, Preussner J, Thomas D, Scholich K, Offermanns S (2018) 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat Commun 9:177. https://doi.org/10.1038/s41467-017-02539-4
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Pontén F (2015) Proteomics Tissue-based map of the human proteome. Science. https://doi.org/10.1126/science.1260419
Veldhuis NA, Lew MJ, Abogadie FC, Poole DP, Jennings EA, Ivanusic JJ, Eilers H, Bunnett NW, McIntyre P (2012) N-glycosylation determines ionic permeability and desensitization of the TRPV1 capsaicin receptor. J Biol Chem 287:21765–72172. https://doi.org/10.1074/jbc.M112.342022
Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA (2001) Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534:813–825. https://doi.org/10.1111/j.1469-7793.2001.00813.x
Vetri F, Saha Roy Choudhury M, Pelligrino DA, Sundivakkam P (2014) BKCa channels as physiological regulators: a focused review. J Receptor Ligand Channel Res. https://doi.org/10.2147/JRLCR.S36065
Vos MH, Neelands TR, McDonald HA, Choi W, Kroeger PE, Puttfarcken PS, Faltynek CR, Moreland RB, Han P (2006) TRPV1b overexpression negatively regulates TRPV1 responsiveness to capsaicin, heat and low pH in HEK293 cells. J Neurochem 99:1088–1102. https://doi.org/10.1111/j.1471-4159.2006.04145.x
Waechter V, Schmid M, Herova M, Weber A, Günther V, Marti-Jaun J, Wüst S, Rösinger M, Gemperle C, Hersberger M (2012) Characterization of the promoter and the transcriptional regulation of the lipoxin A4 receptor (FPR2/ALX) gene in human monocytes and macrophages. J Immunol 188:1856–1867. https://doi.org/10.4049/jimmunol.1101788
Watanabe T, Shimizu T, Miki I, Sakanaka C, Honda Z, Seyama Y, Teramoto T, Matsushima T, Ui M, Kurokawa K (1990) Characterization of the guinea pig lung membrane leukotriene D4 receptor solubilized in an active form. association and dissociation with an islet-activating protein-sensitive guanine nucleotide-binding protein. J Biol Chem 265:21237–21241
Wauquier F, Philippe C, Léotoing L, Mercier S, Davicco MJ, Lebecque P, Guicheux J, Pilet P, Miot-Noirault E, Poitout V, Alquier T, Coxam V, Wittrant Y (2013) The free fatty acid receptor G protein-coupled receptor 40 (GPR40) protects from bone loss through inhibition of osteoclast differentiation. J Biol Chem 288:6542–6551. https://doi.org/10.1074/jbc.M112.429084
Wen H, Östman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A (2012) 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J Biol Chem 287:13868–13876. https://doi.org/10.1074/jbc.M111.334896
Williams-Bey Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, Kehrl JH (2014) Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE 9:e97957. https://doi.org/10.1371/journal.pone.0097957
Wittamer V, Grégoire F, Robberecht P, Vassart G, Communi D, Parmentier M (2004) The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem 279:9956–9962. https://doi.org/10.1074/jbc.M313016200
Woszczek G, Chen LY, Nagineni S, Alsaaty S, Harry A, Logun C, Pawliczak R, Shelhamer JH (2007) IFN-gamma induces cysteinyl leukotriene receptor 2 expression and enhances the responsiveness of human endothelial cells to cysteinyl leukotrienes. J Immunol 178:5262–5270. https://doi.org/10.4049/jimmunol.178.8.5262
Woszczek G, Chen LY, Nagineni S, Kern S, Barb J, Munson PJ, Logun C, Danner RL, Shelhamer JH (2008) Leukotriene D(4) induces gene expression in human monocytes through cysteinyl leukotriene type I receptor. J Allergy Clin Immunol 121:215-221.e1. https://doi.org/10.1016/j.jaci.2007.09.013
Xue Y, Liu Z, Cao J, Ma Q, Gao X, Wang Q, Jin C, Zhou Y, Wen L, Ren J (2011) GPS 2.1: enhanced prediction of kinase-specific phosphorylation sites with an algorithm of motif length selection. Protein Eng Des Sel 24:255–260. https://doi.org/10.1093/protein/gzq094
Yan D, Stocco R, Sawyer N, Nesheim ME, Abramovitz M, Funk CD (2011) Differential signaling of cysteinyl leukotrienes and a novel cysteinyl leukotriene receptor 2 (CysLT2) agonist, N-methyl-leukotriene C4, in calcium reporter and β arrestin assays. Mol Pharmacol 79:270–278. https://doi.org/10.1124/mol.110.069054
Yang F, Zhang Y, Ren H, Wang J, Shang L, Liu Y, Zhu W, Shi X (2019) Ischemia reperfusion injury promotes recurrence of hepatocellular carcinoma in fatty liver via ALOX12-12HETE-GPR31 signaling axis. J Exp Clin Cancer Res 38:489. https://doi.org/10.1186/s13046-019-1480-9
Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA (2009) Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 284:12328–12338. https://doi.org/10.1074/jbc.M806516200
Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T (1997) A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387:620–624. https://doi.org/10.1038/42506
Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T (2000) A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med 192:421–432. https://doi.org/10.1084/jem.192.3.421
Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T (2001) Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem 276:12454–12459. https://doi.org/10.1074/jbc.M011361200
Yokomizo T, Nakamura M, Shimizu T (2018) Leukotriene receptors as potential therapeutic targets. J Clin Invest 128:2691–2701. https://doi.org/10.1172/JCI97946
Yu M, Alonso-Galicia M, Sun CW, Roman RJ, Ono N, Hirano H, Ishimoto T, Reddy YK, Katipally KR, Reddy KM, Gopal VR, Yu J, Takhi M, Falck JR (2003) 20-hydroxyeicosatetraenoic acid (20-HETE): structural determinants for renal vasoconstriction. Bioorg Med Chem 11:2803–2821. https://doi.org/10.1016/s0968-0896(03)00192-5
Zhang C, Booz GW, Yu Q, He X, Wang S, Fan F (2018) Conflicting roles of 20-HETE in hypertension and renal end organ damage. Eur J Pharmacol 833:190–200. https://doi.org/10.1016/j.ejphar.2018.06.010
Zhou JX, Liao D, Zhang S, Cheng N, He HQ, Ye RD (2014) Chemerin C9 peptide induces receptor internalization through a clathrin-independent pathway. Acta Pharmacol Sin 35:653–663. https://doi.org/10.1038/aps.2013.198
Zingoni A, Rocchi M, Storlazzi CT, Bernardini G, Santoni A, Napolitano M (1997) Isolation and chromosomal localization of GPR31, a human gene encoding a putative G protein-coupled receptor. Genomics 42:519–523. https://doi.org/10.1006/geno.1997.4754
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Biringer, R.G. A review of non-prostanoid, eicosanoid receptors: expression, characterization, regulation, and mechanism of action. J. Cell Commun. Signal. 16, 5–46 (2022). https://doi.org/10.1007/s12079-021-00630-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-021-00630-6