Abstract
Objective
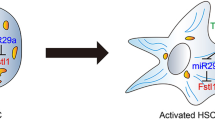
Cleavage of fibronectin type III domain-containing protein 5 (FNDC5), a membrane-bound precursor protein, would cleave into a myokine, irisin, which is also expressed in the liver. FNDC5/Irisin has been reported to play a critical role in maintaining glucose and lipid homeostasis in the liver and in combating liver fibrosis. Recently, several studies have shown that extracellular vesicles (EVs) derived from hepatic stellate cells (HSCs) could modulate liver fibrosis; however, there is a large gap in understanding whether inhibition of fibrogenic EVs derived from HSCs could alleviate the progression of liver fibrosis. Here, we investigated the role of FNDC5/irisin in liver fibrosis and the mechanism of its inhibitory role in the release of HSC-derived fibrogenic EVs.
Methods
Experiments were performed in wild-type and FNDC5−/− mice, primary mouse HSCs, and human hepatic stellate cell line (LX2). Mice were treated with carbon tetrachloride (CCl4) or bile duct ligation (BDL) to induce liver fibrosis. EVs derived from HSCs were purified and injected intraperitoneally into mice.
Results
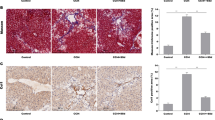
Our results showed that FNDC5 deficiency exacerbated CCl4-induced liver fibrosis and activation of HSCs in mice. Moreover, fibrogenic EVs derived from PDGF-BB-treated HSCs promoted HSC migration in vitro and liver fibrosis in vivo. However, administration of irisin, a cleavage of FNDC5, inhibited the release of fibrogenic EVs and activation of HSCs by promoting ubiquitylation degradation of Rab27b. In vivo, the promoting role of HSC-derived fibrogenic EVs in liver fibrosis was also reversed by irisin.
Conclusion
All these results demonstrate that FNDC5/irisin is a novel therapeutic agent for chronic liver fibrosis.







Similar content being viewed by others
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761
D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68(3):563–576
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397–411
Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456
Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14(8):455–466
Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73(5):1144–1154
Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, et al. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology. 2018;68(1):333–348
Li X, Chen R, Kemper S, Brigstock DR. Dynamic changes in function and proteomic composition of extracellular vesicles from hepatic stellate cells during cellular activation. Cells. 2020;9(2):290
Wan L, Xia T, Du Y, Liu J, Xie Y, Zhang Y, et al. Exosomes from activated hepatic stellate cells contain GLUT1 and PKM2: a role for exosomes in metabolic switch of liver nonparenchymal cells. FASEB J. 2019;33(7):8530–8542
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468
Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta. 2015;1852(9):1867–1875
Zhou B, Ling L, Zhang F, Liu TY, Zhou H, Qi XH, et al. Fibronectin type III domain-containing 5 attenuates liver fibrosis via inhibition of hepatic stellate cell activation. Cell Physiol Biochem. 2018;48(1):227–236
Mederacke I, Dapito DH, Affo S, Uchinami H, Schwabe RF. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat Protoc. 2015;10(2):305–315
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968-977
Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45(3):419–428
Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30 (Suppp 11-13)
Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, et al. Detection and quantitation of circulating human Irisin by Tandem mass spectrometry. Cell Metab. 2015;22(4):734–740
Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61(12):1725–1738
Vaughan RA, Gannon NP, Barberena MA, Garcia-Smith R, Bisoffi M, Mermier CM, et al. Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab. 2014;16(8):711–718
Hou N, Han F, Sun X. The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin Endocrinol. 2015;83(3):339–343
Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769-778
Yan B, Shi X, Zhang H, Pan L, Ma Z, Liu S, et al. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE. 2014;9(4): e94235
Duran ID, Gulcelik NE, Unal M, Topcuoglu C, Sezer S, Tuna MM, et al. Irisin levels in the progression of diabetes in sedentary women. Clin Biochem. 2015;48(18):1268–1272
Espes D, Lau J, Carlsson PO. Increased levels of irisin in people with long-standing Type 1 diabetes. Diabet Med. 2015;32(9):1172–1176
Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592(5):1091–1107
Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications. 2013;27(4):365–369
Xiang L, Xiang G, Yue L, Zhang J, Zhao L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis. 2014;235(2):328–333
Liu TY, Xiong XQ, Ren XS, Zhao MX, Shi CX, Wang JJ, et al. FNDC5 alleviates hepatosteatosis by restoring AMPK/mTOR-mediated autophagy, fatty acid oxidation, and lipogenesis in mice. Diabetes. 2016;65(11):3262–3275
Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127(1):55–64
Park MJ, Kim DI, Choi JH, Heo YR, Park SH. New role of irisin in hepatocytes: the protective effect of hepatic steatosis in vitro. Cell Signal. 2015;27(9):1831–1839
Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, et al. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology. 2016;64(6):2219–2233
Ela S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357
Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64(3):651–660
Funding
Doctor Start-up Fund of Affiliated Hospital of Guizhou Medical University, gyfybsky-2022–15; Science and Technology Planning Project of Guizhou Province (Basic of Guizhou Science and Technology Cooperation -ZK[2023] Key Project[038]).
Author information
Authors and Affiliations
Contributions
Drafting of the manuscript: XL. Research conception and design: FG, YL. Data analysis and interpretation: WS, XN. Statistical analysis: XL, YL. Literature retrieval: FG, XL. Critical revision of the manuscript: QY. Approval of the final manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
Xin Liao, Yilin Luo, Fang Gu, Wen Song, Xin Nie, Qin Yang declare that they have no competing interests.
Ethical approval
Protocols for in vivo experiments were approved by the Animal Welfare and Use Committee of Guizhou Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, X., Luo, Y., Gu, F. et al. Therapeutic role of FNDC5/irisin in attenuating liver fibrosis via inhibiting release of hepatic stellate cell-derived exosomes. Hepatol Int 17, 1659–1671 (2023). https://doi.org/10.1007/s12072-023-10523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-023-10523-y