Abstract
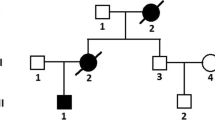
Pediatric restrictive cardiomyopathy (RCM) is the rarest in its group and accounts for only 2.5–5% of all the diagnosed cardiomyopathies in children. It is a relentless disease with poor prognosis, and heart transplantation is the only long-term treatment option. The aetiology of pediatric RCM varies and includes conditions such as endomyocardial fibrosis, storage disorder (Fabry’s disease, MPS), drugs, radiation, post-cardiac transplantation and genetic. Genetic causes encompasses mutations in sarcomeric (troponin I and T, actin, myosin and titin) and nonsarcomeric protein-coding genes (Desmin, RSK2, lamin A/C and bcl-2-associated athanogene 3 (BAG3)). Inheritance of RCM could be autosomal dominant, autosomal recessive and X-linked. Here, we report a case of RCM in an adolescent girl, who was symptomatic with palpitations and breathlessness on exertion. The patient showed presence of rare variants in FLNC (c.5707G>A; p.Glu1903Lys) and BAG3 genes (c.610G>A; p.Gly204Arg). These two variants were detected individually in asymptomatic father and mother, respectively. FLNC gene codes for gamma filamin. These filamin proteins play important role in maintaining the structural integrity of the sarcomere. BAG3 is the main component of the chaperone-assisted selective autophagy (CASA) pathway. Mutant FLNC leads to the formation of protein aggregates which are cleared by an active protein quality control system including CASA pathway. For further verification, in silico protein–protein interaction was performed using online software and tools. The results showed evident interaction between FLNC and BAG3 with significant binding score (−826.6) between them.












Similar content being viewed by others
References
Berman H. M., Westbrook J., Feng Z., Gilliland G., Bhat T. N., Weissig H. et al. 2000 The protein data bank. Nucleic Acid Res. 28, 235–242.
Comeau S. R., Gatchell D. W., Vajda S. and Camacho C. J. 2004a ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 20, 45–50.
Comeau S. R., Gatchell D. W., Vajda S. and Camacho C. J. 2004b ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acid Res. 32, W96–W99.
Dalakas M. C., Park K. Y., Semino Mora C., Lee H. S., Sivakumar K. and Goldfarb L. G. 2000 Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N. Engl. J. Med. 342, 770–780.
Denfield S. W., Rosenthal G., Gajarski R. J., Bricker J. T., Schowengerdt K. O., Price J. K. et al. 1997 Restrictive cardiomyopathies in childhood. Etiologies and natural history. Tex. Heart Inst. J. 24, 38–44.
Finsterer J. and Kothari S. 2014 Cardiac manifestations of primary mitochondrial disorders. Int. J. Cardiol. 177, 754–763.
Kaski J. P., Syrris P., Burch M., Tomé-Esteban M. T., Fenton M., Christiansen M. et al. 2008 Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart 94, 478–484.
Kostareva A., Kiselev A., Gudkova A., Frishman G., Ruepp A., Frishman D. et al. 2016 Genetic spectrum of idiopathic restrictive cardiomyopathy uncovered by next-generation sequencing. PLoS One 11, e0163362.
Kozakov D., Brenke R., Comeau S. R. and Vajda S. 2006 PIPER: an FFT-based protein docking program with pairwise potentials. Proteins 65, 392–406.
Kozakov D., Beglov D., Bohnuud T., Mottarella S. E., Xia B., Hall D. R. et al. 2013 How good is automated protein docking? Proteins 81, 2159–2166.
Lensink M. F. and Wodak S. J. 2013 Docking, scoring, and affinity prediction in CAPRI. Prot. Stru. Fun. Bioinfo. 81, 2082–2095.
Mėlinytė-Ankudavičė K., Šukys M., Plisienė J., Jurkevičius R. and Ereminienė E. 2022 Genotype-phenotype correlation in familial BAG3 mutation dilated cardiomyopathy. Genes (Basel). 13, 363.
Richard P., Charron P., Carrier L., Ledeuil C., Cheav T., Pichereau C. et al. 2003 Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107, 2227–2232.
Scarpini G., Valentino M. L., Giannotta M., Ragni L., Torella A., Columbaro M. et al. 2021 BAG3-related myofibrillar myopathy: a further observation with cardiomyopathy at onset in pediatric age. Acta. Myol. 40, 177–183.
Selcen D., Muntoni F., Burton B. K., Pegoraro E., Sewry C., Bite A. V. et al. 2009 Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann. Neurol. 65, 83–89.
Sherman M. Y. and Gabai V. 2022 The role of Bag3 in cell signaling. J. Cell Biochem. 123, 43–53.
Stürner E. and Behl C. 2017 The role of the multifunctional BAG3 protein in cellular protein quality control and in disease. Front. Mol. Neurosci. 10, 177.
Tucker N. R., McLellan M. A., Hu D., Ye J., Parsons V. A., Mills R. W. et al. 2017 Novel mutation in FLNC (Filamin C) causes familial restrictive cardiomyopathy. Circ. Cardiovasc. Genet. 10, e001780.
Ulbricht A., Eppler F. J., Tapia V. E., Van der Ven P. F. M., Hampe N., Hersch N. et al. 2013 Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr. Biol. 23, 430–435.
Zhou A. X., Hartwig J. H. and Akyürek L. M. 2010 Filamins in cell signaling, transcription and organ development. Tren. Cell Biol. 20, 113–123.
Acknowledgements
Authors acknowledge the support from patient and her family.
Author information
Authors and Affiliations
Corresponding author
Additional information
Coressponding editor: Indrajit Nanda
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, V., Kumar, P., Chauhan, L. et al. Novel combination of FLNC (c.5707G>A; p. Glu1903Lys) and BAG3 (c.610G>A; p.Gly204Arg) genetic variant expressing restrictive cardiomyopathy phenotype in an adolescent girl. J Genet 101, 54 (2022). https://doi.org/10.1007/s12041-022-01402-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12041-022-01402-w