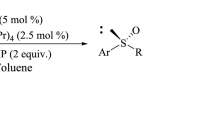
Abstract
While ortho-alkoxy aryl sulfoxides including various substituents were synthesized by Sharpless asymmetric oxidation reaction, we optimized the reaction conditions and screened better combination of starting materials to obtain high enantioselectivity. The result suggested new information that electron-withdrawing substituents on the aromatic ring have a strong influence upon enantioselectivity of the products. Also, several chiral ligands for Sharpless asymmetric oxidation reaction were evaluated to improve the enantioselectivity.
Graphic abstract
High enantioselectivity of ortho-alkoxy aryl chiral sulfoxides have been achieved by Sharpless oxidation reaction using Ti(O-i-Pr)4 and diethyl tartrate under anhydrous condition. In particular, the enantioselctivity of products was influenced by electron-withdrawing substituents on the aromatic ring, such as nitro, ester and aldehyde groups.










Similar content being viewed by others
References
Trost B M and Rao M 2015 Development of chiral sulfoxide ligands for asymmetric catalysis Angew. Chem. Int. Ed. 54 5026
Pulis A P and Procter D J 2016 C-H Coupling reactions directed by sulfoxides: teaching an old functional group new tricks Angew. Chem. Int. Ed. 55 9842
Pellissier H 2006 Use of chiral sulfoxides in asymmetric synthesis Tetrahedron 62 5559
Fernández I, Valdiva V, Leal M P and Khiar N 2007 C2-Symmtric bissulfoxides as organocatalysts in the allylation of benzoyl hydrazones: spacer and concentration effects Org. Lett. 9 2215
Otocka S, Kwiatkowska M, Madalińska L and Kiełbasiński P 2017 Chiral organosulfur ligands/catalysts with a stereogenic sulfur atom: applications in asymmetric synthesis Chem. Rev. 117 4147
Äbelö A, Andersson T B, Antonsson M, Naudot A K, Skånberg I and Weidolf L 2000 Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes Drug Metab. Dispos. 28 966
Flynn G A and Ash R J 1983 Necessity of the sulfoxide moiety for the biochemical and biological properties of an analog of sparsomycin Biochem. Biophys. Res. 114 1
Hansen J L, Schmeing T M, Moore P B and Steitz T A 2002 Structural insights into peptide bond formation PNAS 99 11670
Bentley R 2005 Role of sulfur chirality in the chemical processes of biology Chem. Soc. Rev. 34 609
Xiong F, Yang B-B, Zhang J and Li L 2018 Enantioseparation, stereochemical assignment and chiral recognition mechanism of sulfoxide-containing drugs Molecules 23 2680
Matsui T, Dekishima Y and Ueda M 2014 Biotechnological production of chiral organic sulfoxides: current state and perspectives Appl. Microbiol. Biotechol. 98 7699
Goundry W R F, Adams B, Benson H, Demeritt J, McKown S, Mulholland K, et al. 2017 Development and scale-up of a biocatalytic process to form a chiral sulfoxide Org. Process. Res. Dev. 21 107
Butler B T, Silvey G, Houston D M, Borcherding D R, Vaughn V L, Mcphail A T, et al. 1992 The resolution, isolation, and pharmacological characterization of the enantiomers of a benzamide containing a chiral sulfoxide Chirality 4 155
Kashiyama E, Todaka T, Odomi M, Tanokura Y, Johnson D B, Yokoi T, et al. 1994 Stereoselective pharmacokinetics and interconversions of flosequinan enantiomers containing chiral sulphoxide in rat Xenobiotica 24 369
Katsuki H, Yagi H, Arimori K, Nakamura C, Nakano M, Katafuchi S, et al. 1996 Determination of R (+)- and S (-)-lansoprazole using chiral stationary-phase liquid chromatography and their enantioselective pharmacokinetics in humans Pharm. Res. 13 611
Donovan J L, Malcolm R J, Markowitz J S and De Vane C L 2003 Chiral analysis of d- and l-modafinil in human serum: application to human pharmacokinetic studies Ther. Drug Monit. 25 197
Delatour P, Benoit E, Besse S and Boukraa A 1991 Comparative enantioselectivity in the sulphoxidation of albendazole in man, dogs and rats Xenobiotica 21 217
Cristofol C, Virkel G, Alvarez L, Sánchez S, Arboix M and Lanusse C 2001 Albendazole sulphoxide enantiomeric ratios in plasma and target tissues after intravenous administration of ricobendazole to cattle J. Vet. Pharmacol. Therap. 24 117
Fernández I and Khiar N 2003 Recent developments in the synthesis and utilization of chiral sulfoxides Chem. Rev. 103 3651
Pitchen P, Duñach E, Deshmukh M N and Kagan H B 1984 An efficient asymmetric oxidation of sulfides to sulfoxides J. Am. Chem. Soc. 106 8188
Zhao S H, Samuel O and Kagan H B 1987 Asymmetric oxidation of sulfides mediated by chiral titanium complexes: mechanistic and synthetic aspects Tetrahedron 43 5135
Kagan H B and Rebiere F 1992 Some routes to chiral sulfoxides with very high enantiomeric excesses Synlett 1992 643
Saitoh M, Kunitomo J, Kimura E, Yamano T, Itoh F and Kori M 2010 Enantioselective synthesis of the novel chiral sulfoxide derivatives as a glycogen synthase kinase 3β inhibitor Chem. Pharm. Bull. 58 1252
Capozzi M A M, Cardellicchio C, Naso F and Tortorella P 2000 Substituted benzene anions as leaving groups in the reaction of sulfinyl derivatives with Grignard reagents: a new and convenient route to dialkyl sulfoxides in high enantiomeric purity J. Org. Chem. 65 2843
Bolm C, Müller P and Harms K 1996 Sulfoximine-titanium reagents in enantioselective trimethylsilylcyanations of aldehydes Acta Chem. Scand. 50 305
Wang P, Chen J, Cun L, Deng J, Zhu J and Liao J 2009 Aryl tert-butyl sulfoxide-promoted highly enantioselective addition of allyltrichlorosilane to aldehydes Org. Biomol. Chem. 7 3741
Lin Z, Gong L, Celik M A, Harms K, Frenking G and Meggers E 2011 Asymmetric coordination chemistry by chiral-auxiliary-mediated dynamic resolution under thermodynamic control Chem. Asian J. 6 474
Mohd A, Anitha T, Reddy K R, Wencel-Delord J and Colobert F 2019 P-Stereogenic phosphonates via dynamic kinetic resolution: a route towards enantiopure tertiary phosphine oxides Eur. J. Org. Chem. 2019 7836
Tang Y, Sun Y, Liu J and Duttwyler S 2016 Facile synthesis of enantioenriched phenol-sulfoxides and their aluminum complexes Org. Bioorg. Chem. 14 5580
Gong L, Lin Z, Harms K and Meggers E 2010 Isomerization-induced asymmetric coordination chemistry: from auxiliary control to asymmetric catalysis Angew. Chem. Int. Ed. 49 7955
Seidel F W, Frieß S, Heinemann F W, Chelouan A, Scheurer A, Grasruck A, et al. 2018 C2-Symmetric (SO)N(SO) sulfoxide pincer complexes of Mg and Pd: Helicity switch by ambidentate S/O-coordination and isolation Organometallics 37 1160
Li Z-Z, Wen A-H, Yao S-Y and Ye B-H 2015 Enantioselective syntheses of sulfoxides in octahedral ruthenium(II) complexes via a chiral-at-metal strategy Inorg. Chem. 54 2726
Li Z-Z, Yao S-Y, Wu J-J and Ye B-H 2014 In situ generation of sulfoxides with predetermined chirality via a structural template with a chiral-at-metal ruthenium complex Chem. Commun. 50 5644
Yao S-Y, Chen X-Y, Ou Y-L and Ye B-H 2017 Chiral recognition and dynamic thermodynamic resolution of sulfoxides by chiral iridium(III) complexes Inorg. Chem. 56 878
Rocaboy R, Anastasiou I and Baudoin O 2019 Redox-Neutral Coupling between Two C(sp3)-H bonds enabled by 1,4-palladium shift for the synthesis of fused heterocycles Chem. Int. Ed. 58 14625
Stephenson G R, Roe C and Sandoe E J 2011 Electrophilic C12 building blocks for alkaloids: 1,1 Iterative organoiron-mediated routes to (±)-lycoramine and (±)-maritidine Eur. J. Org. Chem. 9 1664
Liu J-G, Naruta Y and Tani F 2007 Synthetic models of the active site of cytochrome c oxidase: influence of tridentate or tetradentate copper chelates bearing a His-Tyr linkage mimic on dioxygen adduct formation by heme/Cu complexes Chem. Eur. J. 13 6365
Carson M , Giese M W and Coghlan M J 2008 An intra/intermolecular Suzuki sequence to Benzopyridyloxepines containing geometrically pure exocyclic tetrasubstituted alkenes Org. Lett. 10 2701
Obaro-Best O, Reed J, Norfadilah A A F B, Monahan R and Sunasee R 2016 Bismuth trichloride-mediated cleavage of phenolic methoxymethyl ethers Synth. Commun. 46 586
Hughes C C and Trauner D 2004 Palladium-catalyzed couplings to nucleophilic heteroarenes: the total synthesis of (-)-frondosin B Tetrahedron 60 9675
Knewtson K E, Rane D and Peterson B R 2018 Targeting fluorescent sensors to endoplasmic reticulum membranes enables detection of peroxynitrite during cellular phagocytosis ACS Chem. Biol. 13 2595
Basabe P, Diego A, Delgado S, Díez D, Marcos I S and Urones J G 2003 Short and efficient synthesis of (+)-subersic acids Tetrahedron 59 9173
Chausset-Boissarie L, Àrvai R, Cumming G R, Besnard C and Kündig E P 2010 Total synthesis of (±)-Vertine with Z-selective RCM as a key step Chem. Commun. 46 6264
Inoue T, Takada M, Suzuki T and Saito K 1994 Process for producing trisubstituted benzene, and intermediate PCT Int. Appl. 10 9424099
Kalgutkar A S, Kozak K R, Crews B C, Hochgesang G P Jr and Marnett K J 1998 Covalent modification of cyclooxygenase-2 (COX-2) by 2-acetoxyphenyl alkyl sulfides, a new class of selective COX-2 inactivators J. Med. Chem. 41 4800
Donner P L, Randolph J T, Krueger A C, Betebenner D A, Hutchinson D K, Liu D, et al. 2009 Uracil derivatives as HCV inhibitors and their preparation, pharmaceutical compositions and use in the treatment of hepatitis C PCT Int. Appl. 25 2009039135
Peng L, Wen Y, Chen Y, Yuan Z, Zhou Y, Cheng X, et al. 2018 Biocatalytic preparation of chiral sulfoxides through asymmetric reductive resolution by methionine sulfoxide reductase A ChemCatChem 10 3284
Jalba A, Régnier N and Ollevier T 2017 Enantioselective aromatic sulfide oxidation and tandem kinetic resolution using aqueous H2O2 and chiral iron-bis(oxazolinyl)bipyridine catalysts Eur. J. Org. Chem. 2017 1628
Oyaizu K, Mikami T, Mitsuhashi F and Tsuchida E 2002 Synthetic routes to polyheteroacenes: characterization of a heterocyclic ladder polymer containing phenoxathiinium-type building blocks Macromolecules 35 67
Lau S Y W and Keay B A 2001 A highly efficient strategy for the synthesis of 3-substituted salicylic acids by either directed ortho-lithiation or halogen-metal exchange of substituted MOM protected phenols followed by carboxylation Can. J. Chem. 79 1541
Mispelaere-Canivet C, Spindler J-F, Perrio S and Beslin P 2005 Pd2(dba)3/Xantphos-catalyzed cross-coupling of thiols and aryl bromides/triflates Tetrahedron 61 5253
Itoh T and Mase T 2004 A general palladium-catalyzed coupling of aryl bromides/triflates and thiols Org. Lett. 6 4587
Nawrot D, Kolenič M, Kuneš J, Kostelansky F, Miletin M, Novakova V and Zimcik P 2018 Transalkylation of alkyl aryl sulfides with alkylating agents Tetrahedron 74 594
Thompson A, Garabatos-Perera J R and Gillis H M 2008 Asymmetric oxidation of 2-(arylsulfenyl)pyrroles Can. J. Chem. 86 676
Brunel J-M, Diter P, Duetsch M and Kagan H B 1995 Highly enantioselective oxidation of sulfides mediated by a chiral titanium complex J. Org. Chem. 60 8086
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takei, T., Takayama, J., Xuan, M. et al. A study of enantioselective syntheses by Sharpless asymmetric oxidation for aryl sulfoxides containing oxygen groups at the ortho position. J Chem Sci 133, 28 (2021). https://doi.org/10.1007/s12039-021-01887-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-01887-5