Abstract
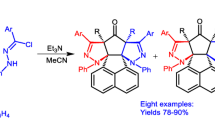
1,3-Dipolar cycloaddition of nitrilimines 3 with N-benzyl maleimide 4 has provided 5-benzyl-1-(2′,4′-dibromophenyl)-3-(4″-substituted phenyl)-3a,4,6,6a-tetrahydro-1H,5H-pyrrolo[3,4-c]pyrazole-4,6-dione derivatives 5 in excellent yield as the only isomer through a concerted pathway.

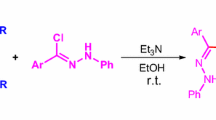
Nitrilimines have been prepared in situ by base catalysed dehydrohalogenation of hydrazonyl halides. Their cycloaddition with N-benzyl maleimides having carbon–carbon double bond has been carried out which leads to the synthesis of 5-benzyl-1-(2′,4′-dibromophenyl)-3-(4″-substituted phenyl)-3a,4,6,6atetrahydro-1H,5H-pyrrolo[3,4-c]pyrazole-4,6-dione derivatives as the single isomer. Stereochemical studies revealed that addition follows cis-endo addition rule.




Similar content being viewed by others
References
Lawyer R W 1963 Chem. Rev. 63 489
Kanemasa S 2002 Synlett 9 1371
Mehta G and Muthusamy S 2002 Tetrahedron 58 9477
Padwa A and Pearson W H 2003 Synthetic applications of 1,3-dipolar cycloaddition chemistry toward heterocycles and natural products (Wiley: New York) vol. 59, p. 952
Koumbis A E and Gallos J K 2003 Curr. Org. Chem. 7 771
Gothelf K V 2002 Cycloaddition reactions in organic synthesis (eds) S Kobayashi and K A Jorgensen (New York: Wiley) chapter 6, p. 211
(a) Huisgen R, Seidel M, Wallbillich G and Knupfer H 1962 Tetrahedron 17 3; (b) Huisgen R 1963 Angew. Chem. Int. Ed. 2 565; (c) Huisgen R, Fliegl W and Kolbeck W 1983 Chem. Ber. 116 3027
Huisgen R, Sustmann R and Wallbillich G 1967 Chem. Ber. 100 1786
Konopikova M, Fisera L and Pronayova N 1991 Collect. Czechoslov. Chem. Commun. 57 1521
Durust Y, Yildirim M, Fronczek C F and Fronczek F R 2012 Monatsh. Chem. 143 127
Huisgen R, Grashey R, Seidel M, Wallbillich G, Knupfer H and Schmidt R 1962 Ann. Chem. 653 105
Fusco R and Romani K 1946 Gazz. Chim. Ital. 76 419
Hassaneen H M, Farg A M, Shawali A S and Alghasib M S 1987 J. Het. Chem. 24 577
Lokanatha Rai K M and Hassner A 1989 Synth. Commun. 19 2799
Huisgen R, Seidel M, Wallbillicher P and Knupfer H 1961 Tetrahedron 17 3
Huisgen R, Seidel M, Sauer J, McFarland W and Wakllbillich G 1959 J. Org. Chem. 24 892
Singh B, Singh B and Krishan K 1999 Indian J. Chem. 38B 93
Badru R, Shah S and Singh B 2011 J. Het. Chem. 49 336
Chattaway F D and Walker A J J 1925 Chem. Soc. 127 975
Acknowledgements
The authors are thankful to the University Grant Commission (UGC), New Delhi, for financial assistance and the Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh and NIPER, Mohali for spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
KAUR, M., SINGH, B. & SINGH, B. 1, 3-Dipolar cycloaddition reactions: Synthesis of 5-benzyl-1-(2′,4′-dibromophenyl)-3-(4″-substituted phenyl)-3a,4,6,6a-tetrahydro-1H, 5H-pyrrolo[3,4-c]pyrazole-4,6-dione derivatives. J Chem Sci 125, 1529–1534 (2013). https://doi.org/10.1007/s12039-013-0526-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0526-3