Abstract
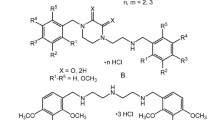
A practical synthesis of (2-butyl-5-nitrobenzofuran-3-yl)(4-hydroxyphenyl)methanone, a key intermediate in the preparation of anti arrhythmic drug, is described. The commercially available 4-nitrophenol (3) is converted in five steps to 2-butyl-5-nitrobenzofuran (9) which upon Friedel–Crafts acylation with 4-methoxybenzoyl chloride followed by deprotection of methyl group gives (2).

An alternative approach for the synthesis of (2-butyl-5-nitrobenzofuran-3-yl)(4-hydroxyphenyl)methanone, a key starting material for dronedarone hydrochloride using commercially available p-nitrophenol as a starting material is described.









Similar content being viewed by others
References
Elkund L 2009 Intl. Patent WO2009/044143
Kretzschmar G, Kraft V, Rossen K and Graser J 2010 Intl. Patent WO2010/136502
Hansson O, Bergh A and Elkund L 2010 Intl. Patent WO2010/116140
Wellig A, Roduit J P, Dai D and Chen R 2010 Intl. Patent WO2010/040261
Elkund L 2010 Intl. Patent WO2010/038029
Bourgeois D, Turconi J and Vastra J 2008 US Patent 2008/0154049
Schouteenten A, Bleger F, Mordacq F and Piron J 2007 US Patent 2007/0155831
Kamal A, Rajasekhar Reddy D, Murali Mohan Reddy P S and Rajendar S 2006 Bioorg. Med. Chem. Lett. 16 1160
Wiles C, Watts P, Haswella S J and Pombo-Villar E 2003 Tetrahedron 59(51) 10173
Wild P G, Wiles C, Watts P and Haswell J S 2009 Tetrahedron 65(8) 1618
Lu J and Xing J 2009 CN 101585813
Joshi R A, Gurjar M K and Tripathy N K 2001 Org. Process Res. Dev. 5(2) 176
Raju B C, Rao N R, Suman P, Yogeeswari P, Sriram D, BShaik B T and Kalivendi S V 2011 Bioorg. Med. Chem. Lett. 21(10) 2855
Sreedhar B, Swapna V and Sridhar Ch 2004 Synth. Commun. 34(8) 1433
Szool T, Brand A and Ratanathanawongs S 1981 J. Chem. Eng. Data. 26(2) 230
Mal D, Jana A, Ray S, Bhattacharya S, Patra A and De S R 2008 Synth. Commun. 38 3937
Bradley T, Honnappa J, Hongmei L I, Sonja S P and Peter D 2005 Intl. Patent WO2005012254
Gao H and Kawabata J 2005 Bioorg. Med. Chem. 13(5) 1661
Larget R, Lockhart B, Renard P and Largeron M 2000 Bioorg. Med. Chem. Lett. 10(8) 835
Ghosh U, Ganessunker D, Sattigeri V J, Carlson K E, Mortensen D J, Katzenellenbogenb B S and Katzenellenbogena J A 2003 Bioorg. Med. Chem. 11 629
Jamode V S and Babrekar S A 2009 Asian J. Chem. 21(5) 3553
(a) Jeanne B A M, Nerina D, Jeanne G F and Olivier M 2010 Intl. Patent WO2010015652; (b) Andreani A, Granaiola M, Leoni A, Locatelli A, Morigi R, Rambaldi M, Lenaz G, Fato R, Bergamini C and Farruggia G 2005 J. Med. Chem. 48(8) 3085
Sudhir S A, Suresh B, Waghmode and Ramaswamy A V 2007 Tetrahedron Lett. 48 1411
Jakhar K and Makrandi J K 2008 Green Chem. Lett. Rev. 1(4) 219
Zhen-Ting D, Jing L, Hong-Rui Y, Yan X and An-Pai L 2010 J. Chem. Res. 34(4) 222
Tsou H R, MacEwan G, Birnberg G, Zhang N, Brooijmans N, Toral-Barza L, Hollander I, Ayral-Kaloustian S and Yu K 2010 Bioorg. Med. Chem. Lett. 20 2259
Zhang N, Ayral-Kaloustian S, James T A, Nguyen T, Das S, Venkatesan A M, N Brooijmans N, Lucas J, Yu K, Hollander I and Mallon R 2010 Bioorg. Med. Chem. Lett. 20 3526
Gutman A, Nisnevich G, Yudovitch L 2007 US Patent 7,312,345 B2
Diouf O, Durand T, Lemeune S, Marcoux J F, Frison N, Larquetoux L and Folleas B 2008 Intl. Patent WO2008/139057
Acknowledgements
The authors wish to thank the management of Dr. Reddy’s Laboratories Ltd., Hyderabad for supporting this work.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RAJA GOPAL, P., CHANDRASHEKAR, E.R.R., SARAVANAN, M. et al. An alternative approach to synthesis of 2-n-butyl-5-nitrobenzofuran derivative: A key starting material for dronedarone hydrochloride. J Chem Sci 124, 1077–1085 (2012). https://doi.org/10.1007/s12039-012-0299-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-012-0299-0