Abstract
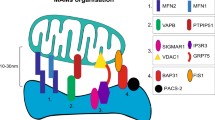
Mitochondria-Endoplasmic Reticulum Contact Sites (MERCS) are dynamic structures whose physiological interaction is vital to direct life and death of the cell. A bevy of tethering proteins, mitofusin-1/2 (Mfn-1/2), glucose-regulated protein-75 (Grp-75), voltage-dependent anion channel-1 (VDAC1), and dynamic-related protein-1 (Drp1), plays an integral role in establishing and regulating this intricate intracellular communication. Dysregulation of this interplay leads to various neurodegenerative disorders, like Alzheimer’s disease (AD), Parkinson’s disease (PD), stroke, traumatic brain injury (TBI), amyotrophic lateral sclerosis (ALS), and frontotemporal dementia (FTD). Although there is an absence of a well-defined molecular background that dictates the pathway of MERCS, adequate exploration has resulted in preliminary data that suggests its cardinal role in neuroregeneration. The juxtaposition of mitochondria and ER has a critical function in cell senescence, thus regulating regeneration. Axonal regeneration and brain tissue regeneration, using reactive astrocytes, are studied most extensively. Overexpression of Grp-75 promoted axonal regeneration post a nerve injury. Attempts have been made to exploit MERCS as potential therapeutic drug targets for enhancing neuroregeneration and impeding neurodegeneration. Novel strategies have been developed to aid the delivery of mitochondria into the neuronal cell body, which in turn establishes a network with the presiding ER resulting in contact site formation. The fascinating aspect of this mechanism is that despite the lack of inherent regenerative capacity in neurons, it can be induced by modifying MERCS.






Similar content being viewed by others
Data Availability
Not applicable.
References
Shirokova OM, Pchelin PV, Mukhina IV (2020) MERCs. The novel assistant to neurotransmission? Front Neurosci. 14:1–14
Giacomello M, Pellegrini L (2016) The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ [Internet] 23(9):1417–27. https://doi.org/10.1038/cdd.2016.52. (Available from)
Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334(6054):358–62
Wu Y, Whiteus C, Xu CS, Hayworth KJ, Weinberg RJ, Hess HF et al (2017) Contacts between the endoplasmic reticulum and other membranes in neurons. Proc Natl Acad Sci U S A 114(24):E4859–E4867
Kornmann B (2020) The endoplasmic reticulum-mitochondria encounter structure: coordinating lipid metabolism across membranes. Biol Chem 401(6–7):811–820
Rowland AA, Voeltz GK (2012) Endoplasmic reticulum–mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13(10):607–625
Wang X, Schwarz TL, Kirby FM (2009) The mechanism of kinesin regulation by Ca ++ for control of mitochondrial motility. Cell 136(1):163–174
Van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9(2):112–124
Fernandes T, Domingues MR, Moreira PI, Pereira CF (2023) A perspective on the link between mitochondria-associated membranes (MAMs) and lipid droplets metabolism in neurodegenerative diseases. Biology (Basel) 12:3
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM et al (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280(5370):1763–6
Csordás G, Várnai P, Golenár T, Roy S, Purkins G, Schneider TG et al (2010) Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39(1):121–132
Saotome M, Safiulina D, Szabadkai G, Das S, Fransson Å, Aspenstrom P et al (2008) Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci U S A 105(52):20728–20733
Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM (2004) Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J Cell Biol 167(1):87–98
Kornmann B, Osman C, Walter P (2011) The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proc Natl Acad Sci U S A 108(34):14151–14156
Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS et al (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325(5939):477–81
Pilling AD, Horiuchi D, Lively CM, Saxton WM (2006) Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in drosophila motor axons. Mol Biol Cell 17:2057–2068
Panda S, Behera S, Alam MF, Syed GH (2021) Endoplasmic reticulum & mitochondrial calcium homeostasis: The interplay with viruses. Mitochondrion 58:227–242
Adachi Y, Kato T, Yamada T, Murata D, Arai K, Stahelin RV et al (2020) Drp1 tubulates the ER in a GTPase independent manner. Mol Cell 80(4):621–632
Tábara LC, Morris JL, Prudent J (2021) The complex dance of organelles during mitochondrial division. Trends Cell Biol 31(4):241–253
Bordoni L, Gabbianelli R (2020) Mitochondrial DNA and neurodegeneration: any role for dietary antioxidants? Antioxidants 9(8):1–24
Lee S, Wang W, Hwang J, Namgung U, Min KT (2019) Increased ER–mitochondria tethering promotes axon regeneration. Proc Natl Acad Sci U S A 116(32):16074–16079
Iadecola C (2017) The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96(1):17–42
Gӧbel J, Engelhardt E, Pelzer P, Sakthivelu V, Jahn HM, Jevtic M et al (2020) Mitochondria-endoplasmic reticulum contacts in reactive astrocytes promote vascular remodeling. Cell Metab 31(4):791-808.e8
Nunes MA, Schöwe NM, Monteiro-Silva KC, Baraldi-Tornisielo T, Souza SIG, Balthazar J et al (2015) Chronic microdose lithium treatment prevented memory loss and neurohistopathological changes in a transgenic mouse model of Alzheimer’s disease. PLoS ONE 10(11):1–26
Rivera AD, Butt AM (2019) Astrocytes are direct cellular targets of lithium treatment: novel roles for lysyl oxidase and peroxisome-proliferator activated receptor-γ as astroglial targets of lithium. Transl Psychiatry. 9:1
Kumar A, Sidhu J, Goyal A (2022) Alzheimer disease. Nih.gov. StatPearls Publishing
Xu L, Wang X, Tong C (2020) Endoplasmic reticulum–mitochondria contact sites and neurodegeneration. Front Cell Dev Biol 18(8):1–12
Adami PVM, Nichtová Z, Weaver DB, Bartok A, Wisniewski T, Jones DR et al (2019) Perturbed mitochondria-ER contacts in live neurons that model the amyloid pathology of Alzheimer’s disease. J Cell Sci. 132:20
Leal NS, Dentoni G, Schreiner B, Naia L, Piras A, Graff C et al (2020) Amyloid Β-peptide increases mitochondria-endoplasmic reticulum contact altering mitochondrial function and autophagosome formation in Alzheimer’s disease-Related Models. Cells 9(12):1–21
Dentoni G, Castro-Aldrete L, Naia L, Ankarcrona M (2022) The potential of small molecules to modulate the mitochondria–endoplasmic reticulum interplay in Alzheimer’s disease. Front Cell Dev Biol 10:1–20
Leal NS, Martins LM (2021) Mind the gap: mitochondria and the endoplasmic reticulum in neurodegenerative diseases. Biomedicines 9(2):1–35
Cieri D, Vicario M, Vallese F, D’Orsi B, Berto P, Grinzato A et al (1864) (2018) Tau localises within mitochondrial sub-compartments and its caspase cleavage affects ER-mitochondria interactions and cellular Ca 2+ handling. Biochim Biophys Acta - Mol Basis Dis [Internet] 10:3247–56. https://doi.org/10.1016/j.bbadis.2018.07.011
Zafar S, Yaddanapudi SS (2019) Parkinson disease Nih.gov. StatPearls Publishing
Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D et al (2019) Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14(1):1–18
Vrijsen S, Vrancx C, Del Vecchio M, Swinnen JV, Agostinis P, Winderickx J et al (2022) Inter-organellar communication in Parkinson’s and Alzheimer’s disease: looking beyond endoplasmic reticulum-mitochondria contact sites. Front Neurosci. [Internet] 21(16):900338. https://doi.org/10.3389/fnins.2022.900338
Calì T, Ottolini D, Negro A, Brini M (1832) (2013) Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca2+ transfer to sustain cell bioenergetics. Biochim Biophys Acta - Mol Basis Dis [Internet] 4:495–508. https://doi.org/10.1016/j.bbadis.2013.01.004. (Available from)
Chang CY, Liang MZ, Chen L (2019) Current progress of mitochondrial transplantation that promotes neuronal regeneration. Transl Neurodegener 8(1):1–12
Rebelo APM, Bello FD, Knedlik T, Kaar N, Volpin F, Shin SH et al (2020) Chemical modulation of mitochondria–endoplasmic reticulum contact sites. Cells 9(7):1637. https://doi.org/10.3390/cells9071637
Eisenberg-Bord M, Shai N, Schuldiner M, Bohnert M (2016) A tether is a tether is a tether: tethering at membrane contact sites. Dev Cell 39(4):395–409
Lau DHW, Paillusson S, Hartopp N, Rupawala H, Mórotz GM, Gomez-Suaga P et al (2020) Disruption of endoplasmic reticulum-mitochondria tethering proteins in post-mortem Alzheimer’s disease brain. Neurobiol Dis. [Internet] 143:105020. https://doi.org/10.1016/j.nbd.2020.105020
Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D et al (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175(6):901–911
Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S et al (2016) Critical reappraisal confirms that mitofusin 2 is an endoplasmic reticulum-mitochondria tether. Proc Natl Acad Sci U S A 113(40):11249–11254
Madreiter-Sokolowski CT, Gottschalk B, Parichatikanond W, Eroglu E, Klec C, Waldeck-Weiermair M et al (2016) Resveratrol specifically kills cancer cells by a devastating increase in the Ca 2+ coupling between the greatly tethered endoplasmic reticulum and mitochondria. Cell Physiol Biochem 39(4):1404–1420
Xie Q, Su J, Jiao B, Shen L, Ma L, Qu X et al (2016) ABT737 reverses cisplatin resistance by regulating ER-mitochondria Ca2+ signal transduction in human ovarian cancer cells. Int J Oncol 49(6):2507–2519
Ardekani AM, Naeini MM (2010) The role of microRNAs in human diseases. Avicenna J Med Biotechnol 2(4):161–179
Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S (2011) Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J 30(3):556–568
Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 160(7):1115–1127
Shi X, Zhao M, Fu C, Fu A (2017) Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion [Internet] 34:91–100. https://doi.org/10.1016/j.mito.2017.02.005. (Available from)
Chang JC, Hoel F, Liu KH, Wei YH, Cheng FC, Kuo SJ et al (2017) Peptide-mediated delivery of donor mitochondria improves mitochondrial function and cell viability in human cybrid cells with the MELAS A3243G mutation. Sci Rep [Internet] 7(1):1–15. https://doi.org/10.1038/s41598-017-10870-5. (Available from)
Chang JC, Chao YC, Chang HS, Wu YL, Chang HJ, Lin YS et al (2021) Intranasal delivery of mitochondria for treatment of Parkinson’s disease model rats lesioned with 6-hydroxydopamine. Sci Rep [Internet] 11(1):1–14. https://doi.org/10.1038/s41598-021-90094-w. (Available from)
Author information
Authors and Affiliations
Contributions
Vijaya Harini Sathyamurthy, Yoghalakshmi Nagarajan and Venkatachalam Deepa Parvathi contributed to the concept, review of literature and manuscript preparation. Vijaya Harini Sathyamurthy and Yoghalakshmi Nagarajan contributed equally towards the draft and figures. Venkatachalam Deepa Parvathi supervised the research work and finalised (review and editing) the manuscript.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sathyamurthy, V.H., Nagarajan, Y. & Parvathi, V.D. Mitochondria-Endoplasmic Reticulum Contact Sites (MERCS): A New Axis in Neuronal Degeneration and Regeneration. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-03971-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-03971-6