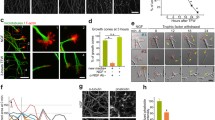
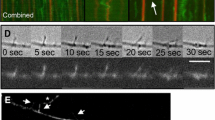
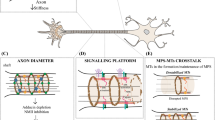
Abstract
Early investigations of the neuronal actin filament cytoskeleton gave rise to the notion that, although growth cones exhibit high levels of actin filaments, the axon shaft exhibits low levels of actin filaments. With the development of new tools and imaging techniques, the axonal actin filament cytoskeleton has undergone a renaissance and is now an active field of research. This article reviews the current state of knowledge about the actin cytoskeleton of the axon shaft. The best understood forms of actin filament organization along axons are axonal actin patches and a submembranous system of rings that endow the axon with protrusive competency and structural integrity, respectively. Additional forms of actin filament organization along the axon have also been described and their roles are being elucidated. Extracellular signals regulate the axonal actin filament cytoskeleton and our understanding of the signaling mechanisms involved is being elaborated. Finally, recent years have seen advances in our perspective on how the axonal actin cytoskeleton is impacted by, and contributes to, axon injury and degeneration. The work to date has opened new venues and future research will undoubtedly continue to provide a richer understanding of the axonal actin filament cytoskeleton.






Similar content being viewed by others
Data Availability
None available as this is a review article.
Abbreviations
- ADF:
-
Actin depolymerizing factor
- AIS:
-
Axon initial segment
- AFB:
-
Actin filament bundles
- ATP:
-
Adenosine triphosphate
- BDNF:
-
Brain-derived neurotrophic factor
- CSPGs:
-
Chondroitin sulfate proteoglycans
- FMN2:
-
Formin-2
- NGF:
-
Nerve growth factor
- PI3K:
-
Phosphoinosite-3 kinase
- PI(3,4,5)P3/PIP3:
-
Phosphatidylinositol (3,4,5)-trisphosphate
- PTEN:
-
Phosphatase and tensin homolog
- PMS:
-
Periodic membrane skeleton
- ROCK:
-
RhoA-kinase
- Sarm1:
-
Sterile alpha and TIR motif containing 1
- Sema3A:
-
Semaphorin 3A
- WAVE1:
-
WASP-family verpolin homologous protein 1
References
Dent EW, Gupton SL, Gertler FB (2011) The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 3(3):a001800
Hoersting AK, Schmucker D (2021) Axonal branch patterning and neuronal shape diversity: roles in developmental circuit assembly. Curr Opin Neurobiol 66:158
Onifer SM, Smith GM, Fouad K (2011) Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics 8(2):283
Lewis TL Jr, Courchet J, Polleux F (2013) Cell biology in neuroscience: cellular and molecular mechanisms underlying axon formation, growth, and branching. J Cell Biol 202(6):837–848
Pollard TD (2017) What we know and do not know about actin. Handb Exp Pharmacol 235:331
Pollard TD (2016) Actin and actin-binding proteins. Cold Spring Harb Perspect Biol 8:a018226
Roy S (2020) Finding order in slow axonal transport. Curr Opin Neurobiol 63:87
Dalla Costa I, Buchanan CN, Zdradzinski MD, Sahoo PK, Smith TP, Thames E, Kar AN, Twiss JL (2021) The functional organization of axonal mRNA transport and translation. Nat Rev Neurosci 22(2):77
Ketschek AR, Jones SL, Gallo G (2007) Axon extension in the fast and slow lanes: substratum-dependent engagement of myosin II functions. Dev Neurobiol 67(10):1305
Turney SG, Bridgman PC (2005) Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat Neurosci 8(6):717
Abosch A, Lagenaur C (1993) Sensitivity of neurite outgrowth to microfilament disruption varies with adhesion molecule substrate. J Neurobiol 24(3):344
Gentile JE, Carrizales MG, Koleske AJ (2022) Control of synapse structure and function by actin and its regulators. Cells 11(4):603
Fath KR, Lasek RJ (1988) Two classes of actin microfilaments are associated with the inner cytoskeleton of axons. J Cell Biol 107(2):613
Chang CM, Goldman RD (1973) The localization of actin-like fibers in cultured neuroblastoma cells as revealed by heavy meromyosin binding. J Cell Biol 57(3):867
LeBeux YJ, Willemot J (1975) An ultrastructural study of the microfilaments in rat brain by means of heavy meromyosin labeling. I. The perikaryon, the dendrites and the axon. Cell Tissue Res 160(1):1
Sanger JW (1975) Intracellular localization of actin with fluorescently labelled heavy meromyosin. Cell Tissue Res 161(4):431
Marchisio PC, Osborn M, Weber K (1978) Changes in intracellular organization of tubulin and actin in N-18 neuroblastoma cells during the process of axon extension induced by serum deprivation. Brain Res 155(2):229
Sotelo J, Toh BH, Yildiz A, Osung O, Holborow EJ (1979) Immunofluorescence demonstrates the distribution of actin, myosin and intermediate filaments in cultured neuroblastoma cells. Neuropathol Appl Neurobiol 5(6):499
Spooner BS, Holladay CR (1981) Distribution of tubulin and actin in neurites and growth cones of differentiating nerve cells. Cell Motil 1(2):167
Goldman JE (1983) Immunocytochemical studies of actin localization in the central nervous system. J Neurosci 3(10):1952
Morris JR, Lasek RJ (1984) Monomer-polymer equilibria in the axon: direct measurement of tubulin and actin as polymer and monomer in axoplasm. J Cell Biol 98(6):2064
Marsh L, Letourneau PC (1984) Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol 99(6):2041
Kobayashi T, Tsukita S, Tsukita S, Yamamoto Y, Matsumoto G (1986) Subaxolemmal cytoskeleton in squid giant axon. I. Biochemical analysis of microtubules, microfilaments, and their associated high-molecular-weight proteins. J Cell Biol. 102(5):1699
Papasozomenos SC, Payne MR (1986) Actin immunoreactivity localizes with segregated microtubules and membraneous organelles and in the subaxolemmal region in the beta, beta′-iminodipropionitrile axon. J Neurosci 6(12):3483
Nagele RG, Kosciuk MC, Hunter ET, Bush KT, Lee H (1988) Immunoelectron microscopic localization of actin in neurites of cultured embryonic chick dorsal root ganglia: actin is a component of granular, microtubule-associated crossbridges. Brain Res 474(2):279
Merino F, Pospich S, Raunser S (2020) Towards a structural understanding of the remodeling of the actin cytoskeleton. Semin Cell Dev Biol 102:51
Xu K, Zhong G, Zhuang X (2013) Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339(6118):452
Vassilopoulos S, Gibaud S, Jimenez A, Caillol G, Leterrier C (2019) Ultrastructure of the axonal periodic scaffold reveals a braid-like organization of actin rings. Nat Commun 10(1):5803
Jones SL, Korobova F, Svitkina T (2014) Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J Cell Biol 205(1):67
Zhong G, He J, Zhou R, Lorenzo D, Babcock HP, Bennett V, Zhuang X (2014) Developmental mechanism of the periodic membrane skeleton in axons. Elife 3:e04581
Qu Y, Hahn I, Webb SE, Pearce SP, Prokop A (2017) Periodic actin structures in neuronal axons are required to maintain microtubules. Mol Biol Cell 28(2):296–308
Lorenzo DN, Edwards RJ, Slavutsky AL (2023) Spectrins: molecular organizers and targets of neurological disorders. Nat Rev Neurosci 24(4):195
Hammarlund M, Jorgensen EM, Bastiani MJ (2007) Axons break in animals lacking beta-spectrin. J Cell Biol 176(3):269
Leite SC, Sampaio P, Sousa VF, Nogueira-Rodrigues J, Pinto-Costa R, Peters LL, Brites P, Sousa MM (2016) The actin-binding protein α-adducin is required for maintaining axon diameter. Cell Rep 15(3):490
Matsuoka Y, Li X, Bennett V (2000) Adducin: structure, function and regulation. Cell Mol Life Sci 57(6):884
Zhou R, Han B, Nowak R, Lu Y, Heller E, Xia C, Chishti AH, Fowler VM et al (2022) Proteomic and functional analyses of the periodic membrane skeleton in neurons. Nat Commun 13(1):3196
Hauser M, Yan R, Li W, Repina NA, Schaffer DV, Xu K (2018) The spectrin-actin-based periodic cytoskeleton as a conserved nanoscale scaffold and ruler of the neural stem cell lineage. Cell Rep 24(6):1512
Lavoie-Cardinal F, Bilodeau A, Lemieux M, Gardner MA, Wiesner T, Laramée G, Gagné C, De Koninck P (2020) Neuronal activity remodels the F-actin based submembrane lattice in dendrites but not axons of hippocampal neurons. Sci Rep 10(1):11960
Dent EW, Gertler FB (2003) Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40(2):209
Hofmann M, Biller L, Michel U, Bähr M, Koch JC (2022) Cytoskeletal assembly in axonal outgrowth and regeneration analyzed on the nanoscale. Sci Rep 12(1):14387
Gomez TM, Snow DM, Letourneau PC (1995) Characterization of spontaneous calcium transients in nerve growth cones and their effect on growth cone migration. Neuron 14(6):1233
Huang CY, Rasband MN (2018) Axon initial segments: structure, function, and disease. Ann N Y Acad Sci 1420(1):46
Hedstrom KL, Ogawa Y, Rasband MN (2008) J AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. Cell Biol 183(4):635
D’Este E, Kamin D, Göttfert F, El-Hady A, Hell SW (2015) STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep 10(8):1246
Berger SL, Leo-Macias A, Yuen S, Khatri L, Pfennig S, Zhang Y, Agullo-Pascual E, Caillol G et al (2018) Localized myosin II activity regulates assembly and plasticity of the axon initial segment. Neuron 97(3):555.e6
Wang T, Li W, Martin S, Papadopulos A, Joensuu M, Liu C, Jiang A, Shamsollahi G et al (2020) Radial contractility of actomyosin rings facilitates axonal trafficking and structural stability. J Cell Biol 219(5):e201902001
Zhang W, Ciorraga M, Mendez P, Retana D, Boumedine-Guignon N, Achón B, Russier M, Debanne D et al (2021) Formin activity and mDia1 contribute to maintain axon initial segment composition and structure. Mol Neurobiol 58(12):6153
Zimmermann D, Homa KE, Hocky GM, Pollard LW, De La Cruz EM, Voth GA, Trybus KM, Kovar DR (2017) Mechanoregulated inhibition of formin facilitates contractile actomyosin ring assembly. Nat Commun 8(1):703
Thiyagarajan S, Wang S, O’Shaughnessy B (2017) A node organization in the actomyosin contractile ring generates tension and aids stability. Mol Biol Cell 28(23):3286
Gallo G (2013) Mechanisms underlying the initiation and dynamics of neuronal filopodia: from neurite formation to synaptogenesis. Int Rev Cell Mol Biol 301:95
Lau PM, Zucker RS, Bentley D (1999) Induction of filopodia by direct local elevation of intracellular calcium ion concentration. J Cell Biol 145(6):1265
Gallo G, Letourneau PC (1998) Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci 18(14):5403
Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G (2011) The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol 71(9):747
Hand RA, Khalid S, Tam E, Kolodkin AL (2015) Axon dynamics during neocortical laminar innervation. Cell Rep 12(2):172
Chen CH, He CW, Liao CP, Pan CL (2017) A Wnt-planar polarity pathway instructs neurite branching by restricting F-actin assembly through endosomal signaling. PLoS Genet 13(4):e1006720
Ketschek A, Gallo G (2010) Nerve growth factor induces axonal filopodia through localized microdomains of phosphoinositide 3-kinase activity that drive the formation of cytoskeletal precursors to filopodia. J Neurosci 30(36):12185
Brosig A, Fuchs J, Ipek F, Kroon C, Schrötter S, Vadhvani M, Polyzou A, Ledderose J et al (2019) The axonal membrane protein PRG2 inhibits PTEN and directs growth to branches. Cell Rep 29(7):2028.e8
Spillane M, Ketschek A, Merianda TT, Twiss JL, Gallo G (2013) Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Rep 5(6):1564
Tao K, Matsuki N, Koyama R (2014) AMP-activated protein kinase mediates activity-dependent axon branching by recruiting mitochondria to axon. Dev Neurobiol 74(6):557
Courchet J, Lewis TL Jr, Lee S, Courchet V, Liou DY, Aizawa S, Polleux F (2013) Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 153(7):1510
Armijo-Weingart L, Ketschek A, Sainath R, Pacheco A, Smith GM, Gallo G (2019) Neurotrophins induce fission of mitochondria along embryonic sensory axons. Elife 8:e49494
Sainath R, Gallo G (2021) Bioenergetic requirements and spatiotemporal profile of nerve growth factor induced PI3K-Akt signaling along sensory axons. Front Mol Neurosci 14:726331
Kundu T, Siva Das S, Sewatkar LK, Kumar DS, Nagar D, Ghose A (2022) Antagonistic activities of Fmn2 and ADF regulate axonal F-actin patch dynamics and the initiation of collateral branching. J Neurosci 42(39):7355
Montaville P, Jégou A, Pernier J, Compper C, Guichard B, Mogessie B, Schuh M, Romet-Lemonne G et al (2014) Spire and Formin 2 synergize and antagonize in regulating actin assembly in meiosis by a ping-pong mechanism. PLoS Biol 12:e1001795
Montaville P, Kühn S, Compper C, Carlier MF (2016) Role of the C-terminal extension of formin 2 in its activation by spire protein and processive assembly of actin filaments. J Biol Chem 291:3302
Spillane M, Ketschek A, Donnelly CJ, Pacheco A, Twiss JL, Gallo G (2012) Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating Arp2/3 complex. J Neurosci 32(49):17671
Loudon RP, Silver LD, Yee HF Jr, Gallo G (2006) RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol 66(8):847
Ketschek A, Spillane M, Dun XP, Hardy H, Chilton J, Gallo G (2016) Drebrin coordinates the actin and microtubule cytoskeleton during the initiation of axon collateral branches. Dev Neurobiol 76(10):1092
Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C (2007) Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development 134(11):2137–2146
Creighton BA, Afriyie S, Ajit D, Casingal CR, Voos KM, Reger J, Burch AM, Dyne E et al (2021) Giant ankyrin-B mediates transduction of axon guidance and collateral branch pruning factor sema 3A. Elife 10:e69815
Chen K, Yang R, Li Y, Zhou JC, Zhang M (2020) Giant ankyrin-B suppresses stochastic collateral axon branching through direct interaction with microtubules. J Cell Biol 219(8):e201910053
Radler MR, Spiliotis ET (2022) Right place, right time - spatial guidance of neuronal morphogenesis by septin GTPases. Curr Opin Neurobiol 75:102557
Hu J, Bai X, Bowen JR, Dolat L, Korobova F, Yu W, Baas PW, Svitkina T et al (2012) Septin-driven coordination of actin and microtubule remodeling regulates the collateral branching of axons. Curr Biol 22(12):1109–1115
Waller TJ, Collins CA (2022) Multifaceted roles of SARM1 in axon degeneration and signaling. Front Cell Neurosci 16:958900
Ketschek A, Holland SM, Gallo G (2022) SARM1 suppresses axon branching through attenuation of axonal cytoskeletal dynamics. Front Mol Neurosci 15:726962
Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB (2012) Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell Rep 2(6):1546
Balasanyan V, Watanabe K, Dempsey WP, Lewis TL Jr, Trinh LA, Arnold DB (2017) Structure and function of an actin-based filter in the proximal axon. Cell Rep 21(10):2696
Lee BH, Bang S, Lee SR, Jeon NL, Park HY (2022) Dynamics of axonal β-actin mRNA in live hippocampal neurons. Traffic 23(10):496
van Bommel B, Konietzny A, Kobler O, Bär J, Mikhaylova M (2019) F-actin patches associated with glutamatergic synapses control positioning of dendritic lysosomes. EMBO J 38(15):e101183
Abouelezz A, Stefen H, Segerstråle M, Micinski D, Minkeviciene R, Lahti L, Hardeman EC, Gunning PW et al (2020) Tropomyosin Tpm3.1 is required to maintain the structure and function of the axon initial segment. iScience 23(5):101053
Mears JA, Ramachandran R (2022) Drp1 and the cytoskeleton: mechanistic nexus in mitochondrial division. Curr Opin Physiol 29:100574
Liu A, Kage F, Higgs HN (2021) Mff oligomerization is required for Drp1 activation and synergy with actin filaments during mitochondrial division. Mol Biol Cell 32(20):ar5
Balzer CJ, Wagner AR, Helgeson LA, Nolen BJ (2019) Single-turnover activation of Arp2/3 complex by Dip1 may balance nucleation of linear versus branched actin filaments. Curr Biol 29(19):3331-3338.e7
Wagner AR, Luan Q, Liu SL, Nolen BJ (2013) Dip1 defines a class of Arp2/3 complex activators that function without preformed actin filaments Curr. Biol 23:1990
Roche FK, Marsick BM, Letourneau PC (2009) Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J Neurosci 29(3):638
Wong HH, Lin JQ, Ströhl F, Roque CG, Cioni JM, Cagnetta R, Turner-Bridger B, Laine RF et al (2017) RNA docking and local translation regulate site-specific axon remodeling in vivo. Neuron 95(4):852.e8
Dent EW, Barnes AM, Tang F, Kalil K (2004) Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci 24(12):3002
Gallo G (2006) RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci 119:3413
Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ (2006) PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci 119(Pt 5):951
Lee H, McKeon RJ, Bellamkonda RV (2010) Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A 107(8):3340
Starkey ML, Bartus K, Barritt AW, Bradbury EJ (2012) Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur J Neurosci 36(12):3665
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298(5596):1248
Sainath R, Ketschek A, Grandi L, Gallo G (2017) CSPGs inhibit axon branching by impairing mitochondria-dependent regulation of actin dynamics and axonal translation. Dev Neurobiol 77(4):454
Silver L, Michael JV, Goldfinger LE, Gallo G (2014) Activation of PI3K and R-Ras signaling promotes the extension of sensory axons on inhibitory chondroitin sulfate proteoglycans. Dev Neurobiol 74(9):918
Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R et al (2011) Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 31(40):14051
Sainath R, Armijo-Weingart L, Ketscheck A, Xu Z, Li S, Gallo G (2017) Chondroitin sulfate proteoglycans negatively regulate the positioning of mitochondria and endoplasmic reticulum to distal axons. Dev Neurobiol 77(12):1351
Kalinski AL, Kar AN, Craver J, Tosolini AP, Sleigh JN, Lee SJ, Hawthorne A, Brito-Vargas P et al (2019) Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. J Cell Biol 218(6):1871
Verburg J, Hollenbeck PJ (2008) Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci 28(33):8306
Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE (2006) Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat Neurosci 9(10):1247
Piper M, Salih S, Weinl C, Holt CE, Harris WA (2005) Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat Neurosci 8(2):179
Chada SR, Hollenbeck PJ (2004) Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol 14(14):1272
Brown JA, Bridgman PC (2009) Disruption of the cytoskeleton during Semaphorin 3A induced growth cone collapse correlates with differences in actin organization and associated binding proteins. Dev Neurobiol 69(10):633
Orlova I, Silver L, Gallo G (2007) Regulation of actomyosin contractility by PI3K in sensory axons. Dev Neurobiol 67(14):1843
Roland AB, Ricobaraza A, Carrel D, Jordan BM, Rico F, Simon A, Humbert-Claude M, Ferrier J et al (2014) Cannabinoid-induced actomyosin contractility shapes neuronal morphology and growth. Elife 3:e03159
Ketschek A, Sainath R, Holland S, Gallo G (2021) The axonal glycolytic pathway contributes to sensory axon extension and growth cone dynamics. J Neurosci 41(31):6637
Ruthel G, Banker G (1998) Actin-dependent anterograde movement of growth-cone-like structures along growing hippocampal axons: a novel form of axonal transport? Cell Motil Cytoskeleton 40(2):160
Flynn KC, Pak CW, Shaw AE, Bradke F, Bamburg JR (2009) Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev Neurobiol 69(12):761
Ruthel G, Banker G (1999) Role of moving growth cone-like “wave” structures in the outgrowth of cultured hippocampal axons and dendrites. J Neurobiol 39(1):97
Mortal S, Iseppon F, Perissinotto A, D’Este E, Cojoc D, Napolitano LMR, Torre V (2017) Actin waves do not boost neurite outgrowth in the early stages of neuron maturation. Front Cell Neurosci 11:402
Iuliano O, Yoshimura A, Prospéri MT, Martin R, Knölker HJ, Coudrier E (2018) Myosin 1b promotes axon formation by regulating actin wave propagation and growth cone dynamics. J Cell Biol 217(6):2033
Tint I, Jean D, Baas PW, Black MM (2009) Doublecortin associates with microtubules preferentially in regions of the axon displaying actin-rich protrusive structures. J Neurosci 29(35):10995
Difato F, Tsushima H, Pesce M, Benfenati F, Blau A, Chieregatti E (2011) The formation of actin waves during regeneration after axonal lesion is enhanced by BDNF. Sci Rep 1:183
Woo D, Seo Y, Jung H, Kim S, Kim N, Park SM, Lee H, Lee S et al (2019) Locally activating TrkB receptor generates actin waves and specifies axonal fate. Cell Chem Biol 26(12):1652.e4
Ganguly A, Tang Y, Wang L, Ladt K, Loi J, Dargent B, Leterrier C, Roy S (2015) A dynamic formin-dependent deep F-actin network in axons. J Cell Biol 210(3):401
Phillips JK, Sherman SA, Cotton KY, Heddleston JM, Taylor AB, Finan JD (2019) Characterization of neurite dystrophy after trauma by high speed structured illumination microscopy and lattice light sheet microscopy. J Neurosci Methods 312:154
Chakrabarty N, Dubey P, Tang Y, Ganguly A, Ladt K, Leterrier C, Jung P, Roy S (2019) Processive flow by biased polymerization mediates the slow axonal transport of actin. J Cell Biol 218(1):112
Schmidt EF, Strittmatter SM (2007) The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol 600:1–11
Gallo G (2004) Myosin II activity is required for severing-induced axon retraction in vitro. Exp Neurol 189(1):112
Shao X, You R, Hui TH, Fang C, Gong Z, Yan Z, Chang RCC, Shenoy VB et al (2019) Tension- and adhesion-regulated retraction of injured axons. Biophys J 117(2):193
Mutalik SP, Joseph J, Pullarkat PA, Ghose A (2018) Cytoskeletal mechanisms of axonal contractility. Biophys J 115(4):713
Tofangchi A, Fan A, Saif MTA (2016) Mechanism of axonal contractility in embryonic drosophila motor neurons in vivo. Biophys J 111(7):1519
Garland P, Broom LJ, Quraishe S, Dalton PD, Skipp P, Newman TA, Perry VH (2012) Soluble axoplasm enriched from injured CNS axons reveals the early modulation of the actin cytoskeleton. PLoS ONE 7(10):e47552
Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S, Yamaguchi A, Mueller BK et al (2008) Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem 105(1):113
Unsain N, Bordenave MD, Martinez GF, Jalil S, von Bilderling C, Barabas FM, Masullo LA, Johnstone AD et al (2018) Remodeling of the actin/spectrin membrane-associated periodic skeleton, growth cone collapse and F-actin decrease during axonal degeneration. Sci Rep 8(1):3007
Sainath R, Gallo G (2015) The dynein inhibitor Ciliobrevin D inhibits the bidirectional transport of organelles along sensory axons and impairs NGF-mediated regulation of growth cones and axon branches. Dev Neurobiol 75(7):757
Koch JC, Tönges L, Barski E, Michel U, Bähr M, Lingor P (2014) ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis 5(5):e1225
Calabrese B, Jones SL, Shiraishi-Yamaguchi Y, Lingelbach M, Manor U, Svitkina TM, Higgs HN, Shih AY et al (2022) INF2-mediated actin filament reorganization confers intrinsic resilience to neuronal ischemic injury. Nat Commun 13(1):6037
Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN (2009) Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci 29(42):13242
Baalman KL, Cotton RJ, Rasband SN, Rasband MN (2013) J Blast wave exposure impairs memory and decreases axon initial segment length. Neurotrauma 30(9):741
Hinman JD, Rasband MN, Carmichael ST (2013) Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke 44(1):182
Bernstein BW, Bamburg JR (2010) ADF/cofilin: a functional node in cell biology. Trends Cell Biol 20(4):187
Bamburg JR, Minamide LS, Wiggan O, Tahtamouni LH, Kuhn TB (2021) Cofilin and actin dynamics: multiple modes of regulation and their impacts in neuronal development and degeneration. Cells 10(10):2726
Bamburg JR, Bernstein BW (2016) Actin dynamics and cofilin-actin rods in Alzheimer disease. Cytoskeleton (Hoboken) 73(9):477
Bamburg JR, Bernstein BW, Davis RC, Flynn KC, Goldsbury C, Jensen JR, Maloney MT, Marsden IT et al (2010) ADF/Cofilin-actin rods in neurodegenerative diseases. Curr Alzheimer Res 7(3):241
Maloney MT, Bamburg JR (2007) Cofilin-mediated neurodegeneration in Alzheimer’s disease and other amyloidopathies. Mol Neurobiol 35(1):21
Hirano A (1994) Hirano bodies and related neuronal inclusions. Neuropathol Appl Neurobiol 20(1):3–11
Galloway PG, Perry G, Gambetti P (1987) Hirano body filaments contain actin and actin-associated proteins. J Neuropathol Exp Neurol 46(2):185–199
Atsumi T, Yamamura Y, Sato T, Ikuta F (1980) Hirano bodies in the axon of peripheral nerves in a case with progressive external ophthalmoplegia with multisystemic involvements. Acta Neuropathol 49(2):95–100
Moradi M, Sivadasan R, Saal L, Lüningschrör P, Dombert B, Rathod RJ, Dieterich DC, Blum R et al (2017) Differential roles of α-, β-, and γ-actin in axon growth and collateral branch formation in motoneurons. J Cell Biol 216(3):793
Donnelly CJ, Park M, Spillane M, Yoo S, Pacheco A, Gomes C, Vuppalanchi D, McDonald M et al (2013) Axonally synthesized β-actin and GAP-43 proteins support distinct modes of axonal growth. J Neurosci 33(8):3311
Gan WB, Kwon E, Feng G, Sanes JR, Lichtman JW (2003) Synaptic dynamism measured over minutes to months: age-dependent decline in an autonomic ganglion. Nat Neurosci 6(9):956–960
Iyengar S, Bottjer SW (2002) Development of individual axon arbors in a thalamocortical circuit necessary for song learning in zebra finches. J Neurosci 22(3):901
Ishida Y, Shirokawa T, Komatsu Y, Isobe K (2001) Changes in cortical noradrenergic axon terminals of locus coeruleus neurons in aged F344 rats. Neurosci Lett 307(3):197
Shirokawa T, Ishida Y, Isobe KI (2000) Age-dependent changes in axonal branching of single locus coeruleus neurons projecting to two different terminal fields. J Neurophysiol 84(2):1120
Pospichal MW, Florence SL, Kaas JH (1994) The postnatal development of geniculocortical axon arbors in owl monkeys. Vis Neurosci 11(1):71
Cowen T (1993) Ageing in the autonomic nervous system: a result of nerve-target interactions? A review. Mech Ageing Dev 68(1–3):163
Kuang RZ, Kalil K (1990) Specificity of corticospinal axon arbors sprouting into denervated contralateral spinal cord. J Comp Neurol 302(3):461
Parekh R, Ascoli GA (2015) Quantitative investigations of axonal and dendritic arbors: development, structure, function, and pathology. Neuroscientist 21(3):241
Chen CH, Chen YC, Jiang HC, Chen CK, Pan CL (2013) Neuronal aging: learning from C. elegans. J Mol Signal 8(1):14
Funding
This work was supported by NIH NINDS R56NS128049 to GG.
Author information
Authors and Affiliations
Contributions
GG wrote the manuscript, reviewed the literature, and prepared the figures.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable. This is a review article.
Consent to Participate
Not applicable. This is a review article.
Consent for Publication
Not applicable. This is a review article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
The axonal actin cytoskeleton serves protrusive and contractile functions.
The axonal actin cytoskeleton forms a ring system to support the plasma membrane.
The axonal actin cytoskeleton protects from axon degeneration.
The axonal actin cytoskeleton is regulated by axonal endomembrane compartments.
The axonal actin cytoskeleton reorganizes during injury and degeneration.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gallo, G. The Axonal Actin Filament Cytoskeleton: Structure, Function, and Relevance to Injury and Degeneration. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-023-03879-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03879-7