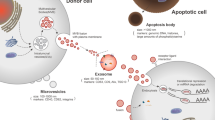
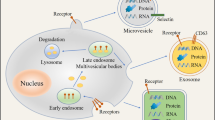
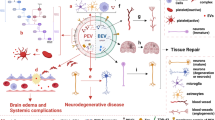
Abstract
CNS (central nervous system) trauma, which is classified as SCI (spinal cord injury) and TBI (traumatic brain injury), is gradually becoming a major cause of accidental death and disability worldwide. Many previous studies have verified that the pathophysiological mechanism underlying cell death and the subsequent neuroinflammation caused by cell death are pivotal factors in the progression of CNS trauma. Simultaneously, EVs (extracellular vesicles), membrane-enclosed particles produced by almost all cell types, have been proven to mediate cell-to-cell communication, and cell death involves complex interactions among molecules. EVs have also been proven to be effective carriers of loaded bioactive components to areas of CNS trauma. Therefore, EVs are promising therapeutic targets to cure CNS trauma. However, the link between EVs and various types of cell death in the context of CNS trauma remains unknown. Therefore, in this review, we summarize the mechanism underlying EV effects, the relationship between EVs and cell death and the pathophysiology underlying EV effects on the CNS trauma based on information in published papers. In addition, we discuss the prospects of applying EVs to the CNS as feasible therapeutic strategies for CNS trauma in the future.




Similar content being viewed by others
Data Availability
Not applicable
Abbreviations
- ATG:
-
Autophagy-related protein
- BBB:
-
Blood–brain barrier
- BMDM:
-
Bone morrow-derived macrophage
- CNS:
-
Central nervous system;
- CSF:
-
Cerebrospinal fluid
- DAMPs:
-
Damage-associated molecular patterns
- ERK:
-
Extracellular signal-regulated kinase
- ESCRT:
-
Endosomal sorting complexes required for transport
- EVs:
-
Extracellular vesicles
- GTPase:
-
Rab guanosine triphosphatase
- ILVs:
-
Intraluminal vesicles
- lncRNA:
-
Long noncoding RNA
- MLKL:
-
Mixed lineage kinase domain-like
- MREs:
-
MicroRNA response elements
- MSC:
-
Mesenchymal stem cell
- MVBs:
-
Multivesicular bodies
- MVs:
-
Microvesicles
- NGF:
-
Nerve growth factor
- NLRP:
-
NOD-like receptor protein
- NSC:
-
Neural stem cell
- OGD:
-
Oxygen-glucose deprivation
- PLD:
-
Phospholipase D
- PS:
-
Phosphatidylserine
- RIPK1:
-
Receptor-interacting protein kinase 1
- RIPK3:
-
Receptor-interacting protein kinase 3
- ROS:
-
Reactive oxygen species
- SCI:
-
Spinal cord injury
- SOCS6:
-
Cytokine signalling 6
- TBI:
-
Traumatic brain injury
- UTR:
-
3′ Untranslated region
References
Dutta D, Khan N, Wu J, Jay SM (2021) Extracellular vesicles as an emerging frontier in spinal cord injury pathobiology and therapy. Trends Neurosci 44(6):492–506. https://doi.org/10.1016/j.tins.2021.01.003
Yates AG, Anthony DC, Ruitenberg MJ, Couch Y (2019) Systemic Immune response to traumatic cns injuries-are extracellular vesicles the missing link? Front Immunol 10:2723. https://doi.org/10.3389/fimmu.2019.02723
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S (2019) Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 10:282. https://doi.org/10.3389/fneur.2019.00282
Pearn ML, Niesman IR, Egawa J, Sawada A, Almenar-Queralt A, Shah SB, Duckworth JL, Head BP (2017) Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell Mol Neurobiol 37(4):571–585. https://doi.org/10.1007/s10571-016-0400-1
Sanwlani R, Gangoda L (2021) Role of extracellular vesicles in cell death and inflammation. Cells 10(10). https://doi.org/10.3390/cells10102663
van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19(4):213–228. https://doi.org/10.1038/nrm.2017.125
Abels ER, Breakefield XO (2016) Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36(3):301–312. https://doi.org/10.1007/s10571-016-0366-z
Kang T, Atukorala I, Mathivanan S (2021) Biogenesis of extracellular vesicles. Subcell Biochem 97:19–43. https://doi.org/10.1007/978-3-030-67171-6_2
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579. https://doi.org/10.1038/nri855
Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW et al (2017) Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal 10(473). https://doi.org/10.1126/scisignal.aai7696
Shi Z, Yuan S, Shi L, Li J, Ning G, Kong X, Feng S (2021) Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif 54(3):e12992. https://doi.org/10.1111/cpr.12992
McDonald JW, Sadowsky C (2002) Spinal-cord injury. Lancet (London, England) 359(9304):417–425. https://doi.org/10.1016/s0140-6736(02)07603-1
Al Mamun A, Wu Y, Monalisa I, Jia C, Zhou K, Munir F, Xiao J (2021) Role of pyroptosis in spinal cord injury and its therapeutic implications. J Adv Res 28:97–109. https://doi.org/10.1016/j.jare.2020.08.004
Luo C, Tao L (2020) The function and mechanisms of autophagy in traumatic brain injury. Adv Exp Med Biol 1207:635–648. https://doi.org/10.1007/978-981-15-4272-5_46
Minciacchi VR, Freeman MR, Di Vizio D (2015) Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 40:41–51. https://doi.org/10.1016/j.semcdb.2015.02.010
Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q (2012) Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A 109(11):4146–4151. https://doi.org/10.1073/pnas.1200448109
Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ (2012) Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods (San Diego, Calif) 56(2):293–304. https://doi.org/10.1016/j.ymeth.2012.01.002
Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J, Chen D (2019) Exosome-induced regulation in inflammatory bowel disease. Front Immunol 10:1464. https://doi.org/10.3389/fimmu.2019.01464
Grant BD, Donaldson JG (2009) Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10(9):597–608. https://doi.org/10.1038/nrm2755
Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL (1991) Late endosomes derive from early endosomes by maturation. Cell 65(3):417–427. https://doi.org/10.1016/0092-8674(91)90459-c
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8(7). https://doi.org/10.3390/cells8070727
Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD (2002) Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell 3(2):271–282. https://doi.org/10.1016/s1534-5807(02)00220-4
de Gassart A, Géminard C, Hoekstra D, Vidal M (2004) Exosome secretion: the art of reutilizing nonrecycled proteins? Traffic (Copenhagen, Denmark) 5(11):896–903. https://doi.org/10.1111/j.1600-0854.2004.00223.x
He C, Zheng S, Luo Y, Wang B (2018) Exosome theranostics: biology and translational medicine. Theranostics 8(1):237–255. https://doi.org/10.7150/thno.21945
Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, Grivel JC (2019) Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer 18(1):55. https://doi.org/10.1186/s12943-019-0965-7
Akers JC, Gonda D, Kim R, Carter BS, Chen CC (2013) Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neuro-Oncol 113(1):1–11. https://doi.org/10.1007/s11060-013-1084-8
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G (2016) Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging 8(4):603–619. https://doi.org/10.18632/aging.100934
Kakarla R, Hur J, Kim YJ, Kim J, Chwae YJ (2020) Apoptotic cell-derived exosomes: messages from dying cells. Exp Mol Med 52(1):1–6. https://doi.org/10.1038/s12276-019-0362-8
Park SJ, Kim JM, Kim J, Hur J, Park S, Kim K, Shin HJ, Chwae YJ (2018) Molecular mechanisms of biogenesis of apoptotic exosome-like vesicles and their roles as damage-associated molecular patterns. Proc Natl Acad Sci U S A 115(50):E11721–E11730. https://doi.org/10.1073/pnas.1811432115
Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS et al (2015) A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun 6:7439. https://doi.org/10.1038/ncomms8439
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A et al (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Controlled Release : Official Journal of the Controlled Release Society 207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033
Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J (2019) Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther 10(1):359. https://doi.org/10.1186/s13287-019-1484-6
Savina A, Vidal M, Colombo MI (2002) The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci 115(Pt 12):2505–2515. https://doi.org/10.1242/jcs.115.12.2505
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO (2020) RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 21(10):585–606. https://doi.org/10.1038/s41580-020-0251-y
Zhu S, Yao F, Qiu H, Zhang G, Xu H, Xu J (2018) Coupling factors and exosomal packaging microRNAs involved in the regulation of bone remodelling. Biological reviews of the Cambridge Philosophical Society 93(1):469–480. https://doi.org/10.1111/brv.12353
Ge X, Tang P, Rong Y, Jiang D, Lu X, Ji C, Wang J, Huang C et al (2021) Exosomal miR-155 from M1-polarized macrophages promotes EndoMT and impairs mitochondrial function via activating NF-κB signaling pathway in vascular endothelial cells after traumatic spinal cord injury. Redox Biol 41:101932. https://doi.org/10.1016/j.redox.2021.101932
Hsu CC, Huang CC, Chien LH, Lin MT, Chang CP, Lin HJ, Chio CC (2020) Ischemia/reperfusion injured intestinal epithelial cells cause cortical neuron death by releasing exosomal microRNAs associated with apoptosis, necroptosis, and pyroptosis. Sci Rep 10(1):14409. https://doi.org/10.1038/s41598-020-71310-5
Joyce DP, Kerin MJ, Dwyer RM (2016) Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int J Cancer 139(7):1443–1448. https://doi.org/10.1002/ijc.30179
Liu S, Chen J, Shi J, Zhou W, Wang L, Fang W, Zhong Y, Chen X et al (2020) M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res Cardiol 115(2):22. https://doi.org/10.1007/s00395-020-0781-7
Teng F, Fussenegger M (2020) Shedding light on extracellular vesicle biogenesis and bioengineering. Adv Sci (Weinheim, Baden-Wurttemberg, Germany) 8(1):2003505. https://doi.org/10.1002/advs.202003505
Levine B, Kroemer G (2019) Biological functions of autophagy genes: a disease perspective. Cell 176(1-2):11–42. https://doi.org/10.1016/j.cell.2018.09.048
Pleet ML, Branscome H, DeMarino C, Pinto DO, Zadeh MA, Rodriguez M, Sariyer IK, El-Hage N et al (2018) Autophagy, EVs, and infections: a perfect question for a perfect time. Front Cell Infect Microbiol 8:362. https://doi.org/10.3389/fcimb.2018.00362
Shao N, Xue L, Wang R, Luo K, Zhi F, Lan Q (2019) miR-454-3p is an exosomal biomarker and functions as a tumor suppressor in glioma. Mol Cancer Ther 18(2):459–469. https://doi.org/10.1158/1535-7163.mct-18-0725
Colletti M, Ceglie D, Di Giannatale A, Nazio F (2020) Autophagy and exosomes relationship in cancer: friends or foes? Front Cell Dev Biol 8:614178. https://doi.org/10.3389/fcell.2020.614178
Ni Z, Kuang L, Chen H, Xie Y, Zhang B, Ouyang J, Wu J, Zhou S et al (2019) The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1β production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis 10(7):522. https://doi.org/10.1038/s41419-019-1739-2
Keller MD, Ching KL, Liang FX, Dhabaria A, Tam K, Ueberheide BM, Unutmaz D, Torres VJ et al (2020) Decoy exosomes provide protection against bacterial toxins. Nature 579(7798):260–264. https://doi.org/10.1038/s41586-020-2066-6
Kuang Y, Zheng X, Zhang L, Ai X, Venkataramani V, Kilic E, Hermann DM, Majid A et al (2020) Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J Extracell Vesicles 10(1):e12024. https://doi.org/10.1002/jev2.12024
Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H (2016) Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology (Baltimore, Md) 64(6):2219–2233. https://doi.org/10.1002/hep.28814
Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, Cooper SA, Cao S et al (2020) Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol 73(5):1144–1154. https://doi.org/10.1016/j.jhep.2020.04.044
Khoury MK, Gupta K, Franco SR, Liu B (2020) Necroptosis in the pathophysiology of disease. Am J Pathol 190(2):272–285. https://doi.org/10.1016/j.ajpath.2019.10.012
Raden Y, Shlomovitz I, Gerlic M (2021) Necroptotic extracellular vesicles - present and future. Semin Cell Dev Biol 109:106–113. https://doi.org/10.1016/j.semcdb.2020.08.011
Gong YN, Guy C, Crawford JC, Green DR (2017) Biological events and molecular signaling following MLKL activation during necroptosis. Cell cycle (Georgetown, Tex) 16(19):1748–1760. https://doi.org/10.1080/15384101.2017.1371889
Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P, Linkermann A, Green DR (2017) ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell 169(2):286–300.e216. https://doi.org/10.1016/j.cell.2017.03.020
Yoon S, Kovalenko A, Bogdanov K, Wallach D (2017) MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 47(1):51–65.e57. https://doi.org/10.1016/j.immuni.2017.06.001
Shlomovitz I, Erlich Z, Arad G, Edry-Botzer L, Zargarian S, Cohen H, Manko T, Ofir-Birin Y et al (2021) Proteomic analysis of necroptotic extracellular vesicles. Cell Death Dis 12(11):1059. https://doi.org/10.1038/s41419-021-04317-z
Abed J, Emgård JE, Zamir G, Faroja M, Almogy G, Grenov A, Sol A, Naor R et al (2016) Fap2 mediates fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 20(2):215–225. https://doi.org/10.1016/j.chom.2016.07.006
Pocanschi CL, Apell HJ, Puntervoll P, Høgh B, Jensen HB, Welte W, Kleinschmidt JH (2006) The major outer membrane protein of Fusobacterium nucleatum (FomA) folds and inserts into lipid bilayers via parallel folding pathways. J Mol Biol 355(3):548–561. https://doi.org/10.1016/j.jmb.2005.10.060
Liu L, Liang L, Yang C, Zhou Y, Chen Y (2021) Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes 13(1):1–20. https://doi.org/10.1080/19490976.2021.1902718
Yuan X, Li D, Chen X, Han C, Xu L, Huang T, Dong Z, Zhang M (2017) Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis 8(12):3200. https://doi.org/10.1038/s41419-017-0041-4
Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42(4):245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VAK (2016) Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165(5):1106–1119. https://doi.org/10.1016/j.cell.2016.04.015
Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458(7242):1191–1195. https://doi.org/10.1038/nature07830
Chavez L, Meguro J, Chen S, de Paiva VN, Zambrano R, Eterno JM, Kumar R, Duncan MR et al (2021) Circulating extracellular vesicles activate the pyroptosis pathway in the brain following ventilation-induced lung injury. J Neuroinflammation 18(1):310. https://doi.org/10.1186/s12974-021-02364-z
Kerr NA, de Rivero Vaccari JP, Umland O, Bullock MR, Conner GE, Dietrich WD, Keane RW (2019) Human lung cell pyroptosis following traumatic brain injury. Cells 8(1). https://doi.org/10.3390/cells8010069
Xu X, Lai Y, Hua ZC (2019) Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep 39(1). https://doi.org/10.1042/bsr20180992
Phan TK, Ozkocak DC, Poon IKH (2020) Unleashing the therapeutic potential of apoptotic bodies. Biochem Soc Trans 48(5):2079–2088. https://doi.org/10.1042/bst20200225
Mesa KR, Rompolas P, Zito G, Myung P, Sun TY, Brown S, Gonzalez DG, Blagoev KB et al (2015) Niche-induced cell death and epithelial phagocytosis regulate hair follicle stem cell pool. Nature 522(7554):94–97. https://doi.org/10.1038/nature14306
Liu J, Qiu X, Lv Y, Zheng C, Dong Y, Dou G, Zhu B, Liu A et al (2020) Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res Ther 11(1):507. https://doi.org/10.1186/s13287-020-02014-w
Liu D, Kou X, Chen C, Liu S, Liu Y, Yu W, Yu T, Yang R et al (2018) Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res 28(9):918–933. https://doi.org/10.1038/s41422-018-0070-2
Ma Q, Liang M, Wu Y, Ding N, Duan L, Yu T, Bai Y, Kang F et al (2019) Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J Biol Chem 294(29):11240–11247. https://doi.org/10.1074/jbc.RA119.007625
Yao X, Zhang Y, Hao J, Duan HQ, Zhao CX, Sun C, Li B, Fan BY, Wang X, Li WX, Fu XH, Hu Y, Liu C, Kong XH, Feng SQ (2019) Deferoxamine promotes recovery of traumatic spinal cord injury by inhibiting ferroptosis. Neural Regen Res 14(3):532–541. https://doi.org/10.4103/1673-5374.245480
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282. https://doi.org/10.1038/s41580-020-00324-8
Zhang X, Xu Y, Ma L, Yu K, Niu Y, Xu X, Shi Y, Guo S, Xue X, Wang Y, Qiu S, Cui J, Wang H, Tian X, Miao Y, Meng F, Qiao Y, Yu Y, Wang J (2022) Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer communications (London, England) 42(4):287–313. https://doi.org/10.1002/cac2.12275
Wu S, Li T, Liu W, Huang Y (2021) Ferroptosis and Cancer: Complex Relationship and Potential Application of Exosomes. Front Cell Dev Biol 9:733751. https://doi.org/10.3389/fcell.2021.733751
Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, Zhang Q, Lin D, Ge S, Bai M, Wang X, Zhang L, Li H, Yang Y, Ji Z, Wang H, Ying G, Ba Y (2020) CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer 19(1):43. https://doi.org/10.1186/s12943-020-01168-8
Zhao X, Si L, Bian J, Pan C, Guo W, Qin P, Zhu W, Xia Y, Zhang Q, Wei K (2022) Adipose tissue macrophage-derived exosomes induce ferroptosis via glutathione synthesis inhibition by targeting SLC7A11 in obesity-induced cardiac injury. Free Radic Biol Med 182:232–245. https://doi.org/10.1016/j.freeradbiomed.2022.02.033
Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, Baer CE, Dixon SJ, Mercurio AM (2019) Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell 51(5):575–586.e574. https://doi.org/10.1016/j.devcel.2019.10.007
Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D (2020) Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 16(11):2069–2083. https://doi.org/10.1080/15548627.2020.1714209
Taylor CA, Bell JM, Breiding MJ, Xu L (2017) Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ (Washington, DC : 2002) 66(9):1–16. https://doi.org/10.15585/mmwr.ss6609a1
Zusman BE, Kochanek PM, Jha RM (2020) Cerebral edema in traumatic brain injury: a historical framework for current therapy. Curr Treat Options Neurol 22:(3). https://doi.org/10.1007/s11940-020-0614-x
Lu J, Ashwell KW, Waite P (2000) Advances in secondary spinal cord injury: role of apoptosis. Spine 25(14):1859–1866
Song B, Zhou T, Liu J, Shao L (2016) Involvement of programmed cell death in neurotoxicity of metallic nanoparticles: recent advances and future perspectives. Nanoscale Res Lett 11(1):484. https://doi.org/10.1186/s11671-016-1704-2
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24(5):254–264. https://doi.org/10.1097/00002826-200109000-00002
Zhou K, Sansur CA, Xu H, Jia X (2017) The temporal pattern, flux, and function of autophagy in spinal cord injury. Int J Mol Sci 18(2). https://doi.org/10.3390/ijms18020466
Feng Z, Min L, Liang L, Chen B, Chen H, Zhou Y, Deng W, Liu H et al (2021) Neutrophil extracellular traps exacerbate secondary injury via promoting neuroinflammation and blood-spinal cord barrier disruption in spinal cord injury. Front Immunol 12:698249. https://doi.org/10.3389/fimmu.2021.698249
Liu Z, Yao X, Jiang W, Li W, Zhu S, Liao C, Zou L, Ding R et al (2020) Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J Neuroinflammation 17(1):90. https://doi.org/10.1186/s12974-020-01751-2
Hao J, Li B, Duan HQ, Zhao CX, Zhang Y, Sun C, Pan B, Liu C et al (2017) Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats. Neural Regen Res 12(6):959–968. https://doi.org/10.4103/1673-5374.208591
Liao HY, Wang ZQ, Ran R, Zhou KS, Ma CW, Zhang HH (2021) Biological functions and therapeutic potential of autophagy in spinal cord injury. Front Cell Dev Biol 9:761273. https://doi.org/10.3389/fcell.2021.761273
Zhou K, Zheng Z, Li Y, Han W, Zhang J, Mao Y, Chen H, Zhang W et al (2020) TFE3, a potential therapeutic target for spinal cord injury via augmenting autophagy flux and alleviating ER stress. Theranostics 10(20):9280–9302. https://doi.org/10.7150/thno.46566
Yuan J, Amin P, Ofengeim D (2019) Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci 20(1):19–33. https://doi.org/10.1038/s41583-018-0093-1
Akamatsu Y, Hanafy KA (2020) Cell death and recovery in traumatic brain injury. Neurotherapeutics : J Am Soc Experimental NeuroTherapeutics 17(2):446–456. https://doi.org/10.1007/s13311-020-00840-7
Yin Y, Sun G, Li E, Kiselyov K, Sun D (2017) ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res Rev 34:3–14. https://doi.org/10.1016/j.arr.2016.08.008
Gao C, Yan Y, Chen G, Wang T, Luo C, Zhang M, Chen X, Tao L (2020) Autophagy activation represses pyroptosis through the IL-13 and JAK1/STAT1 pathways in a mouse model of moderate traumatic brain injury. ACS Chem Neurosci 11(24):4231–4239. https://doi.org/10.1021/acschemneuro.0c00517
Liu ZM, Chen QX, Chen ZB, Tian DF, Li MC, Wang JM, Wang L, Liu BH, Zhang SQ, Li F, Ye H, Zhou L (2018) RIP3 deficiency protects against traumatic brain injury (TBI) through suppressing oxidative stress, inflammation and apoptosis: Dependent on AMPK pathway. Biochem Biophys Res Commun 499(2):112–119. https://doi.org/10.1016/j.bbrc.2018.02.150
Liu S, Li Y, Choi HMC, Sarkar C, Koh EY, Wu J, Lipinski MM (2018) Lysosomal damage after spinal cord injury causes accumulation of RIPK1 and RIPK3 proteins and potentiation of necroptosis. Cell Death Dis 9(5):476. https://doi.org/10.1038/s41419-018-0469-1
Hu X, Xu Y, Zhang H, Li Y, Wang X, Xu C, Ni W, Zhou K (2022) Role of necroptosis in traumatic brain and spinal cord injuries. J Adv Res 40:125–134. https://doi.org/10.1016/j.jare.2021.12.002
Xu X, Yin D, Ren H, Gao W, Li F, Sun D, Wu Y, Zhou S, Lyu L, Yang M, Xiong J, Han L, Jiang R, Zhang J (2018) Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Dis 117:15–27. https://doi.org/10.1016/j.nbd.2018.05.016
Zhang H, Ni W, Yu G, Geng Y, Lou J, Qi J, Chen Y, Li F, Ye H, Ma H, Xu H, Zhao L, Cai Y, Wang X, Xu H, Xiao J, Zhou K (2023) 3,4-Dimethoxychalcone, a caloric restriction mimetic, enhances TFEB-mediated autophagy and alleviates pyroptosis and necroptosis after spinal cord injury. Theranostics 13 (2):810–832. https://doi.org/10.7150/thno.78370
Liao HY, Wang ZQ, Ran R, Zhou KS, Ma CW, Zhang HH (2021) Biological Functions and Therapeutic Potential of Autophagy in Spinal Cord Injury. Front Cell Dev Biol 9:761273. https://doi.org/10.3389/fcell.2021.761273
Zhang L, Wang H (2018) Autophagy in Traumatic Brain Injury: A New Target for Therapeutic Intervention. Front Mol Neurosci 11:190. https://doi.org/10.3389/fnmol.2018.00190
Wong J, Hoe NW, Zhiwei F, Ng I (2005) Apoptosis and traumatic brain injury. Neurocrit Care 3(2):177–182. https://doi.org/10.1385/ncc:3:2:177
Xu G, Zhang J, Wang L, Cui Z, Sun X, Liu Z, Zhu Z, Qiu Y (2017) Up-regulation of TRAF2 Suppresses Neuronal Apoptosis after Rat Spinal Cord Injury. Tissue & cell 49(5):589–596. https://doi.org/10.1016/j.tice.2017.08.002
Feng Z, Min L, Chen H, Deng W, Tan M, Liu H, Hou J (2021) Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol 43:101984. https://doi.org/10.1016/j.redox.2021.101984
Xie BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, Jiang JY (2019) Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther 25(4):465–475. https://doi.org/10.1111/cns.13069
Gupta K, Brown KA, Hsieh ML, Hoover BM, Wang J, Khoury MK, Pilli VSS, Beyer RSH, Voruganti NR, Chaudhary S, Roberts DS, Murphy RM, Hong S, Ge Y, Liu B (2022) Necroptosis is associated with Rab27-independent expulsion of extracellular vesicles containing RIPK3 and MLKL. J Extracell Vesicles 11(9):e12261. https://doi.org/10.1002/jev2.12261
Khellaf A, Khan DZ, Helmy A (2019) Recent advances in traumatic brain injury. J Neurol 266(11):2878–2889. https://doi.org/10.1007/s00415-019-09541-4
Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR (2017) Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant 26(7):1118–1130. https://doi.org/10.1177/0963689717714102
Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB (2014) Transcranial amelioration of inflammation and cell death after brain injury. Nature 505(7482):223–228. https://doi.org/10.1038/nature12808
Nekludov M, Mobarrez F, Gryth D, Bellander BM, Wallen H (2014) Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J Neurotrauma 31(23):1927–1933. https://doi.org/10.1089/neu.2013.3168
Kuharić J, Grabušić K, Tokmadžić VS, Štifter S, Tulić K, Shevchuk O, Lučin P, Šustić A (2019) Severe traumatic brain injury induces early changes in the physical properties and protein composition of intracranial extracellular vesicles. J Neurotrauma 36(2):190–200. https://doi.org/10.1089/neu.2017.5515
Beard K, Meaney DF, Issadore D (2020) Clinical applications of extracellular vesicles in the diagnosis and treatment of traumatic brain injury. J Neurotrauma 37(19):2045–2056. https://doi.org/10.1089/neu.2020.6990
Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV, Farhoodi HP, Zhang SX et al (2016) Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9(4):509–529. https://doi.org/10.1007/s12195-016-0458-3
van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M (2001) Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121(2):337–349. https://doi.org/10.1053/gast.2001.26263
Beard K, Yang Z, Haber M, Flamholz M, Diaz-Arrastia R, Sandsmark D, Meaney DF, Issadore D (2021) Extracellular vesicles as distinct biomarker reservoirs for mild traumatic brain injury diagnosis. Brain Comm 3(3):fcab151. https://doi.org/10.1093/braincomms/fcab151
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC et al (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287(6):3842–3849. https://doi.org/10.1074/jbc.M111.277061
Kondo K, Maruishi M, Ueno H, Sawada K, Hashimoto Y, Ohshita T, Takahashi T, Ohtsuki T et al (2010) The pathophysiology of prospective memory failure after diffuse axonal injury--lesion-symptom analysis using diffusion tensor imaging. BMC Neurosci 11:147. https://doi.org/10.1186/1471-2202-11-147
Werner JK, Stevens RD (2015) Traumatic brain injury: recent advances in plasticity and regeneration. Curr Opin Neurol 28(6):565–573. https://doi.org/10.1097/wco.0000000000000265
Kabadi SV, Faden AI (2014) Neuroprotective strategies for traumatic brain injury: improving clinical translation. Int J Mol Sci 15(1):1216–1236. https://doi.org/10.3390/ijms15011216
Mondello S, Thelin EP, Shaw G, Salzet M, Visalli C, Cizkova D, Kobeissy F, Buki A (2018) Extracellular vesicles: pathogenetic, diagnostic and therapeutic value in traumatic brain injury. Expert Rev Proteomics 15(5):451–461. https://doi.org/10.1080/14789450.2018.1464914
Budden CF, Gearing LJ, Kaiser R, Standke L, Hertzog PJ, Latz E (2021) Inflammasome-induced extracellular vesicles harbour distinct RNA signatures and alter bystander macrophage responses. J Extracell Vesicles 10(10):e12127. https://doi.org/10.1002/jev2.12127
Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, Anantharam V, Huang X et al (2019) Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal 12(563). https://doi.org/10.1126/scisignal.aat9900
Qu Y, Franchi L, Nunez G, Dubyak GR (2007) Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179(3):1913–1925. https://doi.org/10.4049/jimmunol.179.3.1913
Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR et al (2017) Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14(1):47. https://doi.org/10.1186/s12974-017-0819-4
Hazelton I, Yates A, Dale A, Roodselaar J, Akbar N, Ruitenberg MJ, Anthony DC, Couch Y (2018) Exacerbation of acute traumatic brain injury by circulating extracellular vesicles. J Neurotrauma 35(4):639–651. https://doi.org/10.1089/neu.2017.5049
Campbell SJ, Zahid I, Losey P, Law S, Jiang Y, Bilgen M, van Rooijen N, Morsali D et al (2008) Liver Kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology 55(5):780–787. https://doi.org/10.1016/j.neuropharm.2008.06.074
Campbell SJ, Hughes PM, Iredale JP, Wilcockson DC, Waters S, Docagne F, Perry VH, Anthony DC (2003) CINC-1 is an acute-phase protein induced by focal brain injury causing leukocyte mobilization and liver injury. FASEB J 17(9):1168–1170. https://doi.org/10.1096/fj.02-0757fje
Vats R, Brzoska T, Bennewitz MF, Jimenez MA, Pradhan-Sundd T, Tutuncuoglu E, Jonassaint J, Gutierrez E et al (2020) Platelet extracellular vesicles drive inflammasome-IL-1β-dependent lung injury in sickle cell disease. Am J Respir Crit Care Med 201(1):33–46. https://doi.org/10.1164/rccm.201807-1370OC
de Rivero Vaccari JP, Brand F 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, Bullock MR, Dietrich WD et al (2016) Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem 136(Suppl 1):39–48. https://doi.org/10.1111/jnc.13036
Xue Y, Enosi Tuipulotu D, Tan WH, Kay C, Man SM (2019) Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol 40(11):1035–1052. https://doi.org/10.1016/j.it.2019.09.005
Zhao P, Cao L, Wang X, Dong J, Zhang N, Li X, Li J, Zhang X et al (2021) Extracellular vesicles secreted by Giardia duodenalis regulate host cell innate immunity via TLR2 and NLRP3 inflammasome signaling pathways. PLoS Negl Trop Dis 15(4):e0009304. https://doi.org/10.1371/journal.pntd.0009304
Wang X, Eagen WJ, Lee JC (2020) Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. Proc Natl Acad Sci U S A 117(6):3174–3184. https://doi.org/10.1073/pnas.1915829117
Anjum A, Yazid MD, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, Ismail OHR, Athi Kumar RK et al (2020) Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci 21(20). https://doi.org/10.3390/ijms21207533
Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L (2017) Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release 262:247–258. https://doi.org/10.1016/j.jconrel.2017.07.001
Upadhya R, Zingg W, Shetty S, Shetty AK (2020) Astrocyte-derived extracellular vesicles: neuroreparative properties and role in the pathogenesis of neurodegenerative disorders. J Control Release 323:225–239. https://doi.org/10.1016/j.jconrel.2020.04.017
Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F (2020) Ferroptosis and cancer: mitochondria meet the “Iron Maiden” cell death. Cells 9(6). https://doi.org/10.3390/cells9061505
Luo Z, Xu X, Sho T, Zhang J, Xu W, Yao J, Xu J (2019) ROS-induced autophagy regulates porcine trophectoderm cell apoptosis, proliferation, and differentiation. Am J Physiol Cell Physiol 316(2):C198–c209. https://doi.org/10.1152/ajpcell.00256.2018
You Y, Borgmann K, Edara VV, Stacy S, Ghorpade A, Ikezu T (2020) Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J Extracell Vesicles 9(1):1706801. https://doi.org/10.1080/20013078.2019.1706801
Datta Chaudhuri A, Dasgheyb RM, DeVine LR, Bi H, Cole RN, Haughey NJ (2020) Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 68(1):128–144. https://doi.org/10.1002/glia.23708
Rong Y, Ji C, Wang Z, Ge X, Wang J, Ye W, Tang P, Jiang D et al (2021) Small extracellular vesicles encapsulating CCL2 from activated astrocytes induce microglial activation and neuronal apoptosis after traumatic spinal cord injury. J Neuroinflammation 18(1):196. https://doi.org/10.1186/s12974-021-02268-y
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. https://doi.org/10.1101/cshperspect.a001651
Zhao W, Ma L, Cai C, Gong X (2019) Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-induced THP-1 macrophages. Int J Biol Sci 15(8):1571–1581. https://doi.org/10.7150/ijbs.34211
An Y, Zhang H, Wang C, Jiao F, Xu H, Wang X, Luan W, Ma F et al (2019) Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J 33(11):12515–12527. https://doi.org/10.1096/fj.201802805RR
Liu Z, Yao X, Sun B, Jiang W, Liao C, Dai X, Chen Y, Chen J et al (2021) Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic Biol Med 168:142–154. https://doi.org/10.1016/j.freeradbiomed.2021.03.037
Smirnov SV, Harbacheuski R, Lewis-Antes A, Zhu H, Rameshwar P, Kotenko SV (2007) Bone-marrow-derived mesenchymal stem cells as a target for cytomegalovirus infection: implications for hematopoiesis, self-renewal and differentiation potential. Virology 360(1):6–16. https://doi.org/10.1016/j.virol.2006.09.017
Keshtkar S, Azarpira N, Ghahremani MH (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther 9(1):63. https://doi.org/10.1186/s13287-018-0791-7
Madrigal M, Rao KS, Riordan NH (2014) A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med 12:260. https://doi.org/10.1186/s12967-014-0260-8
Phinney DG, Pittenger MF (2017) Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells (Dayton, Ohio) 35(4):851–858. https://doi.org/10.1002/stem.2575
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33(11):1711–1715. https://doi.org/10.1038/jcbfm.2013.152
Reza-Zaldivar EE, Hernández-Sapiéns MA, Minjarez B, Gutiérrez-Mercado YK, Márquez-Aguirre AL, Canales-Aguirre AA (2018) Potential effects of MSC-derived exosomes in neuroplasticity in Alzheimer’s disease. Front Cell Neurosci 12:317. https://doi.org/10.3389/fncel.2018.00317
Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D et al (2019) Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano 13(9):10015–10028. https://doi.org/10.1021/acsnano.9b01892
Williams AM, Dennahy IS, Bhatti UF, Halaweish I, Xiong Y, Chang P, Nikolian VC, Chtraklin K et al (2019) Mesenchymal stem cell-derived exosomes provide neuroprotection and improve long-term neurologic outcomes in a Swine model of traumatic brain injury and hemorrhagic shock. J Neurotrauma 36(1):54–60. https://doi.org/10.1089/neu.2018.5711
Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS (2011) Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res 108(11):1340–1347. https://doi.org/10.1161/circresaha.110.239848
Rong Y, Liu W, Wang J, Fan J, Luo Y, Li L, Kong F, Chen J et al (2019) Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis 10(5):340. https://doi.org/10.1038/s41419-019-1571-8
Lawson A, Snyder W, Peeples ES (2022) Intranasal administration of extracellular vesicles mitigates apoptosis in a mouse model of neonatal hypoxic-ischemic brain injury. Neonatology 119:1–9. https://doi.org/10.1159/000522644
Pei X, Li Y, Zhu L, Zhou Z (2019) Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp Cell Res 382(2):111474. https://doi.org/10.1016/j.yexcr.2019.06.019
Chen W, Wang H, Zhu Z, Feng J, Chen L (2020) Exosome-shuttled circSHOC2 from IPASs regulates neuronal autophagy and ameliorates ischemic brain injury via the miR-7670-3p/SIRT1 axis. Mol Ther Nucleic Acids 22:657–672. https://doi.org/10.1016/j.omtn.2020.09.027
Pei X, Li Y, Zhu L, Zhou Z (2020) Astrocyte-derived exosomes transfer miR-190b to inhibit oxygen and glucose deprivation-induced autophagy and neuronal apoptosis. Cell Cycle 19(8):906–917. https://doi.org/10.1080/15384101.2020.1731649
Chen Y, Li J, Ma B, Li N, Wang S, Sun Z, Xue C, Han Q et al (2020) MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 12(18):18274–18296. https://doi.org/10.18632/aging.103692
Song Y, Wang B, Zhu X, Hu J, Sun J, Xuan J, Ge Z (2021) Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol 37(1):51–64. https://doi.org/10.1007/s10565-020-09530-8
Lin F, Chen W, Zhou J, Zhu J, Yao Q, Feng B, Feng X, Shi X et al (2022) Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis 13(3):271. https://doi.org/10.1038/s41419-022-04708-w
Hu Z, Yuan Y, Zhang X, Lu Y, Dong N, Jiang X, Xu J, Zheng D (2021) Human umbilical cord mesenchymal stem cell-derived exosomes attenuate oxygen-glucose deprivation/reperfusion-induced microglial pyroptosis by promoting FOXO3a-dependent mitophagy. Oxidative Med Cell Longev 2021:6219715. https://doi.org/10.1155/2021/6219715
Zhang B, Lin F, Dong J, Liu J, Ding Z, Xu J (2021) Peripheral macrophage-derived exosomes promote repair after spinal cord injury by inducing local anti-inflammatory type microglial polarization via increasing autophagy. Int J Biol Sci 17(5):1339–1352. https://doi.org/10.7150/ijbs.54302
Wang J, Rong Y, Ji C, Lv C, Jiang D, Ge X, Gong F, Tang P et al (2020) MicroRNA-421-3p-abundant small extracellular vesicles derived from M2 bone marrow-derived macrophages attenuate apoptosis and promote motor function recovery via inhibition of mTOR in spinal cord injury. J Nanobiotechnol 18(1):72. https://doi.org/10.1186/s12951-020-00630-5
Huang J, Cao H, Cui B, Ma X, Gao L, Yu C, Shen F, Yang X, Liu N, Qiu A, Cai G, Zhuang S (2022) Mesenchymal Stem Cells-Derived Exosomes Ameliorate Ischemia/Reperfusion Induced Acute Kidney Injury in a Porcine Model. Front Cell Dev Biol 10:899869. https://doi.org/10.3389/fcell.2022.899869
Merkle FT, Alvarez-Buylla A (2006) Neural stem cells in mammalian development. Curr Opin Cell Biol 18(6):704–709. https://doi.org/10.1016/j.ceb.2006.09.008
Xiong LL, Hu Y, Zhang P, Zhang Z, Li LH, Gao GD, Zhou XF, Wang TH (2018) Neural stem cell transplantation promotes functional recovery from traumatic brain injury via brain derived neurotrophic factor-mediated neuroplasticity. Mol Neurobiol 55(3):2696–2711. https://doi.org/10.1007/s12035-017-0551-1
Fukuoka T, Kato A, Hirano M, Ohka F, Aoki K, Awaya T, Adilijiang A, Sachi M et al (2021) Neurod4 converts endogenous neural stem cells to neurons with synaptic formation after spinal cord injury. iScience 24(2):102074. https://doi.org/10.1016/j.isci.2021.102074
De Gioia R, Biella F, Citterio G, Rizzo F, Abati E, Nizzardo M, Bresolin N, Comi GP et al (2020) Neural stem cell transplantation for neurodegenerative diseases. Int J Mol Sci 21(9). https://doi.org/10.3390/ijms21093103
Koprivec S, Novak M, Bernik S, Voga M, Mohorič L, Majdič G (2021) Treatment of cranial cruciate ligament injuries in dogs using a combination of tibial tuberosity advancement procedure and autologous mesenchymal stem cells/multipotent mesenchymal stromal cells - a pilot study. Acta Vet Hung 68(4):405–412. https://doi.org/10.1556/004.2020.00063
Li WY, Zhu QB, Jin LY, Yang Y, Xu XY, Hu XY (2021) Exosomes derived from human induced pluripotent stem cell-derived neural progenitor cells protect neuronal function under ischemic conditions. Neural Regen Res 16(10):2064–2070. https://doi.org/10.4103/1673-5374.308665
Milich LM, Ryan CB, Lee JK (2019) The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol 137(5):785–797. https://doi.org/10.1007/s00401-019-01992-3
Zhang C, Li D, Hu H, Wang Z, An J, Gao Z, Zhang K, Mei X et al (2021) Engineered extracellular vesicles derived from primary M2 macrophages with anti-inflammatory and neuroprotective properties for the treatment of spinal cord injury. J Nanobiotechnol 19(1):373. https://doi.org/10.1186/s12951-021-01123-9
Mills CD, Shearer J, Evans R, Caldwell MD (1992) Macrophage arginine metabolism and the inhibition or stimulation of cancer. J Immunol 149(8):2709–2714
Wang L, Chopp M, Szalad A, Lu X, Zhang Y, Wang X, Cepparulo P, Lu M et al (2020) Exosomes derived from Schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes 69(4):749–759. https://doi.org/10.2337/db19-0432
Wu X, Wang L, Cong M, Shen M, He Q, Ding F, Shi H (2020) Extracellular vesicles from skin precursor-derived Schwann cells promote axonal outgrowth and regeneration of motoneurons via Akt/mTOR/p70S6K pathway. Ann Transl Med 8(24):1640. https://doi.org/10.21037/atm-20-5965
Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S, Zhou Z, Rong Y et al (2020) Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnol 18(1):105. https://doi.org/10.1186/s12951-020-00665-8
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32(6):2003–2014. https://doi.org/10.1007/s11095-014-1593-y
Yang Y, Ye Y, Su X, He J, Bai W, He X (2017) MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front Cell Neurosci 11:55. https://doi.org/10.3389/fncel.2017.00055
Cocucci E, Meldolesi J (2015) Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25(6):364–372. https://doi.org/10.1016/j.tcb.2015.01.004
Long X, Yao X, Jiang Q, Yang Y, He X, Tian W, Zhao K, Zhang H (2020) Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J Neuroinflammation 17(1):89. https://doi.org/10.1186/s12974-020-01761-0
Huang S, Ge X, Yu J, Han Z, Yin Z, Li Y, Chen F, Wang H et al (2018) Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J 32(1):512–528. https://doi.org/10.1096/fj.201700673R
Yin Z, Han Z, Hu T, Zhang S, Ge X, Huang S, Wang L, Yu J et al (2020) Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun 83:270–282. https://doi.org/10.1016/j.bbi.2019.11.004
Zhang Y, Zhang Y, Chopp M, Pang H, Zhang ZG, Mahmood A, Xiong Y (2021) MiR-17-92 Cluster-enriched exosomes derived from human bone marrow mesenchymal stromal cells improve tissue and functional recovery in rats after traumatic brain injury. J Neurotrauma 38(11):1535–1550. https://doi.org/10.1089/neu.2020.7575
Li D, Huang S, Zhu J, Hu T, Han Z, Zhang S, Zhao J, Chen F et al (2019) Exosomes from MiR-21-5p-increased neurons play a role in neuroprotection by suppressing Rab11a-mediated neuronal autophagy in vitro after traumatic brain injury. Med Sci Monit 25:1871–1885. https://doi.org/10.12659/msm.915727
He B, Chen W, Zeng J, Tong W, Zheng P (2021) Long noncoding RNA NKILA transferred by astrocyte-derived extracellular vesicles protects against neuronal injury by upregulating NLRX1 through binding to mir-195 in traumatic brain injury. Aging 13(6):8127–8145. https://doi.org/10.18632/aging.202618
Patel NA, Moss LD, Lee JY, Tajiri N, Acosta S, Hudson C, Parag S, Cooper DR et al (2018) Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J Neuroinflammation 15(1):204. https://doi.org/10.1186/s12974-018-1240-3
Chen J, Zhang C, Li S, Li Z, Lai X, Xia Q (2021) Exosomes derived from nerve stem cells loaded with FTY720 promote the recovery after spinal cord injury in rats by PTEN/AKT signal pathway. J Immunol Res 2021:8100298. https://doi.org/10.1155/2021/8100298
Han T, Song P, Wu Z, Xiang X, Liu Y, Wang Y, Fang H, Niu Y et al (2022) MSC secreted extracellular vesicles carrying TGF-beta upregulate Smad 6 expression and promote the regrowth of neurons in spinal cord injured rats. Stem Cell Rev Rep 18(3):1078–1096. https://doi.org/10.1007/s12015-021-10219-6
Jia X, Huang G, Wang S, Long M, Tang X, Feng D, Zhou Q (2021) Extracellular vesicles derived from mesenchymal stem cells containing microRNA-381 protect against spinal cord injury in a rat model via the BRD4/WNT5A axis. Bone Joint Res 10(5):328–339. https://doi.org/10.1302/2046-3758.105.bjr-2020-0020.r1
Jiang Z, Zhang J (2021) Mesenchymal stem cell-derived exosomes containing miR-145-5p reduce inflammation in spinal cord injury by regulating the TLR4/NF-κB signaling pathway. Cell Cycle 20(10):993–1009. https://doi.org/10.1080/15384101.2021.1919825
Yang Y, Ye Y, Kong C, Su X, Zhang X, Bai W, He X (2019) MiR-124 enriched exosomes promoted the m2 polarization of microglia and enhanced hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res 44(4):811–828. https://doi.org/10.1007/s11064-018-02714-z
Yao X, Liu S, Ding W, Yue P, Jiang Q, Zhao M, Hu F, Zhang H (2017) TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. J Neuroimmunol 310:38–45. https://doi.org/10.1016/j.jneuroim.2017.06.006
Xiao X, Jiang Y, Liang W, Wang Y, Cao S, Yan H, Gao L, Zhang L (2019) miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol Brain 12(1):78. https://doi.org/10.1186/s13041-019-0501-0
Harrison EB, Hochfelder CG, Lamberty BG, Meays BM, Morsey BM, Kelso ML, Fox HS, Yelamanchili SV (2016) Traumatic brain injury increases levels of miR-21 in extracellular vesicles: implications for neuroinflammation. FEBS open bio 6(8):835–846. https://doi.org/10.1002/2211-5463.12092
Li D, Huang S, Yin Z, Zhu J, Ge X, Han Z, Tan J, Zhang S et al (2019) Increases in miR-124-3p in microglial exosomes confer neuroprotective effects by targeting FIP200-mediated neuronal autophagy following traumatic brain injury. Neurochem Res 44(8):1903–1923. https://doi.org/10.1007/s11064-019-02825-1
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441):333–338. https://doi.org/10.1038/nature11928
Guo S, Redenski I, Levenberg S (2021) Spinal cord repair: from cells and tissue engineering to extracellular vesicles. Cells 10(8). https://doi.org/10.3390/cells10081872
Sas AR, Carbajal KS, Jerome AD, Menon R, Yoon C, Kalinski AL, Giger RJ, Segal BM (2020) A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat Immunol 21(12):1496–1505. https://doi.org/10.1038/s41590-020-00813-0
Ohori Y, Yamamoto S, Nagao M, Sugimori M, Yamamoto N, Nakamura K, Nakafuku M (2006) Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci 26(46):11948–11960. https://doi.org/10.1523/jneurosci.3127-06.2006
Betzer O, Perets N, Angel A, Motiei M, Sadan T, Yadid G, Offen D, Popovtzer R (2017) In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano 11(11):10883–10893. https://doi.org/10.1021/acsnano.7b04495
Zhang D, Ni N, Wang Y, Tang Z, Gao H, Ju Y, Sun N, He X et al (2021) CircRNA-vgll3 promotes osteogenic differentiation of adipose-derived mesenchymal stem cells via modulating miRNA-dependent integrin α5 expression. Cell Death Differ 28(1):283–302. https://doi.org/10.1038/s41418-020-0600-6
Song Y, Zhang C, Zhang J, Jiao Z, Dong N, Wang G, Wang Z, Wang L (2019) Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 9(8):2346–2360. https://doi.org/10.7150/thno.29945
de Abreu RC, Fernandes H, da Costa Martins PA, Sahoo S, Emanueli C, Ferreira L (2020) Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol 17(11):685–697. https://doi.org/10.1038/s41569-020-0389-5
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM (2015) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205:35–44. https://doi.org/10.1016/j.jconrel.2014.11.029
Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N (2016) Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol 79:360–369. https://doi.org/10.1016/j.biocel.2016.09.002
Kooijmans SAA, Fliervoet LAL, van der Meel R, Fens M, Heijnen HFG, PMP v BEH, Vader P, Schiffelers RM (2016) PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release 224:77–85. https://doi.org/10.1016/j.jconrel.2016.01.009
Acknowledgements
Figures 1–3 were illustrated by using Figdraw (www.figdraw.com).
Funding
This work was funded by grants from the National Natural Science Foundation of China (No. 82072192 to Kailiang Zhou); Wenzhou Science and Technology Bureau Foundation (No. Y20210438 to Kailiang Zhou); Public Welfare Technology Application Research Project of Zhejiang Province (LGF20H150003 to Kailiang Zhou); and Zhejiang Provincial Natural Science Foundation (No. LY17H060009 to Wenfei Ni).
Author information
Authors and Affiliations
Contributions
Yituo Chen, Haojie Zhang and Xinli Hu searched and reviewed literature and drafted manuscript and revision; Wanta Cai and Liting Jiang discussed and revised the manuscript; Yongli Wang, Yanqing Wu and Xiangyang Wang provided critical comments. Kailiang Zhou and Wenfei Ni designed and formulated the review theme and revised and finalized the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
All authors consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Zhang, H., Hu, X. et al. Extracellular Vesicles: Therapeutic Potential in Central Nervous System Trauma by Regulating Cell Death. Mol Neurobiol 60, 6789–6813 (2023). https://doi.org/10.1007/s12035-023-03501-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03501-w