Abstract
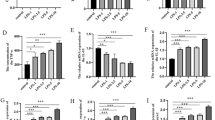
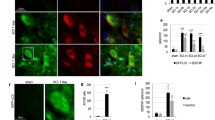
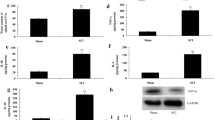
There are limited therapeutic options for patient with traumatic spinal cord injury (SCI). Phosphoinositide 3-kinase family (PI3Ks) are the key molecules for regulating cell autophagy, which is a possible way of treating SCI. As we know, PI3K family are composed of eight isoforms, which are distributed into three classes. While the role of PI3Ks in regulating autophagy is controversial and the effects may be in a cell-specific manner. Different isoforms do not distribute in neural cells consistently and it is not clear how the PI3K isoforms regulate and interact with autophagy. Therefore, we explored the distributions and expression of different PI3K isoforms in two key neural cells (PC12 cells and astrocytes). The results showed that the expression of LC3II/I and p62, which are the markers of autophagy, changed in different patterns in PC12 cells and astrocytes after hypoxia/reoxygenation injury (H/R). Furthermore, the mRNA level of eight PI3K isoforms did not change in the same way, and even for the same isoform the mRNA activities are different between PC12 cells and astrocytes. What is more, the results of western blot of PI3K isoforms after H/R were inconsistent with the relevant mRNA. Based on this study, the therapeutic effects of regulating autophagy on SCI are not confirmed definitely, and its molecular mechanisms may be related with different temporal and spatial patterns of activation and distributions of PI3K isoforms.







Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Zhang D, Zhu D, Wang F, Zhu J-C, Zhai X, Yuan Y, Li C-X (2020) Therapeutic effect of regulating autophagy in spinal cord injury: a network meta-analysis of direct and indirect comparisons. Neural Regen Res 15(6):1120–1132. https://doi.org/10.4103/1673-5374.270419
Smith E, Fitzpatrick P, Murtagh J, Lyons F, Morris S, Synnott K (2018) Epidemiology of traumatic spinal cord injury in Ireland, 2010-2015. Neuroepidemiology 51(1-2):19–24. https://doi.org/10.1159/000488146
Chan BC-F, Cadarette SM, Wodchis WP, Krahn MD, Mittmann N (2019) The lifetime cost of spinal cord injury in Ontario, Canada: a population-based study from the perspective of the public health care payer. J Spinal Cord Med 42(2):184–193. https://doi.org/10.1080/10790268.2018.1486622
Ullah MM, Fossey E, Stuckey R (2018) The meaning of work after spinal cord injury: a scoping review. Spinal Cord 56(2):92–105. https://doi.org/10.1038/s41393-017-0006-6
Elshahidi MH, Monir NY, Elzhery MA, Sharaqi AA, Haedaya H, Awad BI, Zaghloul K (2018) Epidemiological characteristics of traumatic spinal cord injury (TSCI) in the Middle-East and North-Africa (MENA) region: a systematic review and meta-analysis. Bull Emerg Trauma 6(2):75–89. https://doi.org/10.29252/beat-060201
Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp 71(2):281–299
Cui C, Cui N, Wang P, Song S, Liang H, Ji A (2015) Sulfated polysaccharide isolated from the sea cucumber stichopus japonicus against PC12 hypoxia/reoxygenation injury by inhibition of the MAPK signaling pathway. Cell Mol Neurobiol 35(8):1081–1092. https://doi.org/10.1007/s10571-015-0202-x
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S (2019) Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 10. https://doi.org/10.3389/fneur.2019.00282
Rong Y, Fan J, Ji C, Wang Z, Ge X, Wang J, Ye W, Yin G et al (2022) USP11 regulates autophagy-dependent ferroptosis after spinal cord ischemia-reperfusion injury by deubiquitinating Beclin 1. Cell Death Differ 29(6):1164–1175. https://doi.org/10.1038/s41418-021-00907-8
Chen F-S, Tong X-Y, Fang B, Wang D, Li X-Q, Zhang Z-L (2022) The roles of microRNAs in spinal cord ischemia-reperfusion injury. Neural Regen Res 17(12):2593–2599. https://doi.org/10.4103/1673-5374.339471
Wu Q, Xiang Z, Ying Y, Huang Z, Tu Y, Chen M, Ye J, Dou H et al (2021) Nerve growth factor (NGF) with hypoxia response elements loaded by adeno-associated virus (AAV) combined with neural stem cells improve the spinal cord injury recovery. Cell Death. Discovery 7(1). https://doi.org/10.1038/s41420-021-00701-y
Zhang D, Yuan Y, Zhu J, Zhu D, Li C, Cui W, Wang L, Ma S et al (2021) Insulin-like growth factor 1 promotes neurological functional recovery after spinal cord injury through inhibition of autophagy via the PI3K/Akt/mTOR signaling pathway. Exp Ther Med 22(5). https://doi.org/10.3892/etm.2021.10700
Zhang Q, Liu M, Nong H, Zhang Y, Bai Y, Liu P, Zong S, Zeng G (2022) Total flavonoids of hawthorn leaves protect spinal motor neurons via promotion of autophagy after spinal cord injury. Front Pharmacol 13. https://doi.org/10.3389/fphar.2022.925568
Huang Y, Ren H, Gao X, Cai D, Shan H, Bai J, Sheng L, Jin Y et al (2022) Amlodipine improves spinal cord injury repair by inhibiting motoneuronal apoptosis through autophagy upregulation. Spine 47(17):E570–E578. https://doi.org/10.1097/brs.0000000000004310
de Almeida FM, Marques SA, Dos Santos ACR, Prins CA, Dos Santos Cardoso FS, Dos Santos HL, Mendonca HR, Martinez AMB (2023) Molecular approaches for spinal cord injury treatment. Neural Regen Res 18(1):23–30. https://doi.org/10.4103/1673-5374.344830
Ray SK (2020) Modulation of autophagy for neuroprotection and functional recovery in traumatic spinal cord injury. Neural Regen Res 15(9):1601–1612. https://doi.org/10.4103/1673-5374.276322
Zhao H, Yang Y, Si X, Liu H, Wang H (2022) The role of pyroptosis and autophagy in ischemia reperfusion injury. Biomolecules 12(7). https://doi.org/10.3390/biom12071010
Talebi M, Vadoud SAM, Haratian A, Talebi M, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S (2022) The interplay between oxidative stress and autophagy: focus on the development of neurological diseases. Behav Brain Funct 18(1). https://doi.org/10.1186/s12993-022-00187-3
Li Y, Lei Z, Ritzel RM, He J, Li H, Choi HMC, Lipinski MM, Wu J (2022) Impairment of autophagy after spinal cord injury potentiates neuroinflammation and motor function deficit in mice. Theranostics 12(12):5364–5388. https://doi.org/10.7150/thno.72713
Filippone A, Esposito E, Mannino D, Lyssenko N, Pratico D (2022) The contribution of altered neuronal autophagy to neurodegeneration. Pharmacol Ther 238. https://doi.org/10.1016/j.pharmthera.2022.108178
Chen D, Li C, Lv R (2021) MicroRNA-218 aggravates H2O2-induced damage in PC12 cells via spred2-mediated autophagy. Exp Ther Med 22(6). https://doi.org/10.3892/etm.2021.10787
Liao H-Y, Wang Z-Q, Ran R, Zhou K-S, Ma C-W, Zhang H-H (2021) Biological functions and therapeutic potential of autophagy in spinal cord injury. Front Cell Dev Biol 9. https://doi.org/10.3389/fcell.2021.761273
Pan D, Zhu S, Zhang W, Wei Z, Yang F, Guo Z, Ning G, Feng S (2022) Autophagy induced by Schwann cell-derived exosomes promotes recovery after spinal cord injury in rats. Biotechnol Lett 44(1):129–142. https://doi.org/10.1007/s10529-021-03198-8
Das CK, Mandal M, Koegel D (2018) Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastasis Rev 37(4):749–766. https://doi.org/10.1007/s10555-018-9727-z
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15(2):81–94. https://doi.org/10.1038/nrm3735
Nakka VP, Prakash-babu P, Vemuganti R (2016) Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol Neurobiol 53(1):532–544. https://doi.org/10.1007/s12035-014-9029-6
Zhou K, Sansur CA, Xu H, Jia X (2017) The temporal pattern, flux, and function of autophagy in spinal cord injury. Int J Mol Sci 18(2). https://doi.org/10.3390/ijms18020466
Jean S, Kiger AA (2014) Classes of phosphoinositide 3-kinases at a glance. Journal of Cell Science 127(5):923–928. https://doi.org/10.1242/jcs.093773
He X, Li Y, Deng B, Lin A, Zhang G, Ma M, Wang Y, Yang Y et al (2022) The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: mechanisms and therapeutic opportunities. Cell Prolif 55(9). https://doi.org/10.1111/cpr.13275
Li J, Cheng X, Fu D, Liang Y, Chen C, Deng W, He L (2022) Autophagy of spinal microglia affects the activation of microglia through the PI3K/AKT/mTOR signaling pathway. Neuroscience 482:77–86. https://doi.org/10.1016/j.neuroscience.2021.12.005
Hassett MR, Sternberg AR, Roepe PD (2017) Inhibition of human class I vs Class III phosphatidylinositol 3 ′-kinases. Biochemistry 56(33):4326–4334. https://doi.org/10.1021/acs.biochem.7b00413
Arcucci S, Ramos-Delgado F, Cayron C, Therville N, Gratacap M-P, Basset C, Thibault B, Guillermet-Guibert J (2021) Organismal roles for the PI3K alpha and beta isoforms: their specificity, redundancy or cooperation is context-dependent. Biochem J 478(6):1199–1225. https://doi.org/10.1042/bcj20210004
Liu X, Wang A, Liang X, Liu J, Zou F, Chen C, Zhao Z, Deng Y et al (2016) Simultaneous inhibition of Vps34 kinase would enhance PI3K delta inhibitor cytotoxicity in the B-cell malignancies. Oncotarget 7(33):53515–53525. https://doi.org/10.18632/oncotarget.10650
Griffey CJ, Yamamoto A (2022) Macroautophagy in CNS health and disease. Nat Rev Neurosci 23(7):411–427. https://doi.org/10.1038/s41583-022-00588-3
Walker CL, Wu X, Liu N-K, Xu X-M (2019) Bisperoxovanadium mediates neuronal protection through inhibition of PTEN and activation of PI3K/AKT-mTOR signaling after traumatic spinal injuries. J Neurotrauma 36(18):2676–2687. https://doi.org/10.1089/neu.2018.6294
Zhang H-Y, Wang Z-G, Wu F-Z, Kong X-X, Yang J, Lin B-B, Zhu S-P, Lin L et al (2013) Regulation of autophagy and ubiquitinated protein accumulation by bFGF promotes functional recovery and neural protection in a rat model of spinal cord injury. Mol Neurobiol 48(3):452–464. https://doi.org/10.1007/s12035-013-8432-8
Zhang J, Yuan L, Wang S, Liu J, Bi H, Chen G, Li J, Chen L (2020) Germacrone protects against oxygen-glucose deprivation/reperfusion injury by inhibiting autophagy processes in PC12 cells. Bmc Complementary Med Ther 20(1). https://doi.org/10.1186/s12906-020-2865-1
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Jin YC, Yang SF, Zhang XH (2017) Reduction of neuronal damage and promotion of locomotor recovery after spinal cord injury by early administration of methylprednisolone: possible involvement of autophagy pathway. Rsc Advances 7(5):2979–2991. https://doi.org/10.1039/c6ra25794a
Karimi-Abdolrezaee S, Billakanti R (2012) Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol Neurobiol 46(2):251–264. https://doi.org/10.1007/s12035-012-8287-4
Anderson MA, Burda JE, Ren YL, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS et al (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532(7598):195-+. https://doi.org/10.1038/nature17623
Okada S, Hara M, Kobayakawa K, Matsumoto Y, Nakashima Y (2018) Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci Res 126:39–43. https://doi.org/10.1016/j.neures.2017.10.004
Sultan N, Amin LE, Zaher AR, Grawish ME, Scheven BA (2021) Dental pulp stem cells stimulate neuronal differentiation of PC12 cells. Neural Regen Res 16(9):1821–1828. https://doi.org/10.4103/1673-5374.306089
Zhong Z, Yao X, Luo M, Li M, Dong L, Zhang Z, Jiang R (2020) Protocatechuic aldehyde mitigates hydrogen peroxide-triggered PC12 cell damage by down-regulating MEG3. Artif Cells Nanomedicine Biotechnol 48(1):602–609. https://doi.org/10.1080/21691401.2020.1725535
Xia N, Gao Z, Hu H, Li D, Zhang C, Mei X, Wu C (2021) Nerve growth factor loaded macrophage-derived nanovesicles for inhibiting neuronal apoptosis after spinal cord injury. J Biomater Appl 36(2):276–288. https://doi.org/10.1177/08853282211025912
Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J (2015) Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis 6. https://doi.org/10.1038/cddis.2014.527
Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L et al (2014) A dual role for autophagy in a murine model of lung cancer. Nat Commun 5. https://doi.org/10.1038/ncomms4056
Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, OFX A, Sousa N (2008) Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3 beta. Neuroscience 152(3):656–669. https://doi.org/10.1016/j.neuroscience.2007.12.026
Zhang D, Wang F, Zhai X, Li XH, He XJ (2018) Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy. Neural Regen Res 13(12):2191–2199. https://doi.org/10.4103/1673-5374.241473
Hou HP, Zhang LH, Zhang LC, Tang PF (2014) Acute spinal cord injury in rats should target activated autophagy. J Neurosurg-Spine 20(5):568–577. https://doi.org/10.3171/2014.1.Spine13237
Wang HY, Wang YS, Li DD, Liu ZY, Zhao ZM, Han DH, Yuan YJ, Bi J et al (2015) VEGF inhibits the inflammation in spinal cord injury through activation of autophagy. Biochem Biophys Res Commun 464(2):453–458. https://doi.org/10.1016/j.bbrc.2015.06.146
Fang B, Li XQ, Bao NR, Tan WF, Chen FS, Pi XL, Zhang Y, Ma H (2016) Role of autophagy in the bimodal stage after spinal cord ischemia reperfusion injury in rats. Neuroscience 328:107–116. https://doi.org/10.1016/j.neuroscience.2016.04.019
Cong Y, Wang CQ, Wang JY, Li HX, Li Q (2020) NT-3 promotes oligodendrocyte proliferation and nerve function recovery after spinal cord injury by inhibiting autophagy pathway. J Surg Res 247:128–135. https://doi.org/10.1016/j.jss.2019.10.033
Wang P, Lin CW, Wu SY, Huang KL, Wang Y, Bao XM, Zhang F, Huang ZH et al (2018) Inhibition of autophagy is involved in the protective effects of ginsenoside Rb1 on spinal cord injury. Cell Mol Neurobiol 38(3):679–690. https://doi.org/10.1007/s10571-017-0527-8
Xie L, Yu S, Yang K, Li C, Liang Y (2017) Hydrogen sulfide inhibits autophagic neuronal cell death by reducing oxidative stress in spinal cord ischemia reperfusion injury. Oxid Med Cell Longev 2017. https://doi.org/10.1155/2017/8640284
Li Y, Guo Y, Fan Y, Tian H, Li K, Mei X (2019) Melatonin enhances autophagy and reduces apoptosis to promote locomotor recovery in spinal cord injury via the PI3K/AKT/mTOR signaling pathway. Neurochem Res 44(8):2007–2019. https://doi.org/10.1007/s11064-019-02838-w
Gulluni F, De Santis MC, Margaria JP, Martini M, Hirsch E (2019) Class II PI3K functions in cell biology and disease. Trends Cell Biol 29(4):339–359. https://doi.org/10.1016/j.tcb.2019.01.001
Valet C, Chicanne G, Severac C, Chaussade C, Whitehead MA, Cabou C, Gratacap MP, Gaits-Iacovoni F et al (2015) Essential role of class II PI3K-C2 alpha in platelet membrane morphology. Blood 126(9):1128–1137. https://doi.org/10.1182/blood-2015-03-636670
Vergne I, Deretic V (2010) The role of PI3P phosphatases in the regulation of autophagy. Febs Letters 584(7):1313–1318. https://doi.org/10.1016/j.febslet.2010.02.054
Bilanges B, Posor Y, Vanhaesebroeck B (2019) PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev Mol Cell Biol 20(9):515–534. https://doi.org/10.1038/s41580-019-0129-z
Aksoy E, Saveanu L, Manoury B (2018) The Isoform selective roles of PI3Ks in dendritic cell biology and function. Front Immunol 9. https://doi.org/10.3389/fimmu.2018.02574
Lupieri A, Smirnova N, Let NM, Gayral S, Laffargue M (2015) PI3K signaling in arterial diseases: non redundant functions of the PI3K isoforms. Advances in Biological. Regulation 59(Sp. Iss. SI):4–18. https://doi.org/10.1016/j.jbior.2015.06.002
Juss JK, Hayhoe RP, Owen CE, Bruce I, Walmsley SR, Cowburn AS, Kulkarni S, Boyle KB et al (2012) Functional redundancy of class I phosphoinositide 3-kinase (PI3K) isoforms in signaling growth factor-mediated human neutrophil survival. Plos One 7(9). https://doi.org/10.1371/journal.pone.0045933
White AR, Tiwari D, MacLeod MC, Danzer SC, Gross C (2020) PI3K isoform-selective inhibition in neuron-specific PTEN-deficient mice rescues molecular defects and reduces epilepsy-associated phenotypes. Neurobiol Dis 144. https://doi.org/10.1016/j.nbd.2020.105026
Hawkins PT (1851) Stephens LR (2015) PI3K signalling in inflammation. Biochim Et Biophysica Acta-Mol Cell Biol Lipids 6:882–897. https://doi.org/10.1016/j.bbalip.2014.12.006
Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT (2017) The PI3K pathway in human disease. Cell 170(4):605–635. https://doi.org/10.1016/j.cell.2017.07.029
Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML, Bilando A, Need AC (et. al, 2007) Control of axonal growth and regeneration of sensory neurons by the p110 delta PI 3-kinase. Plos One 2(9). https://doi.org/10.1371/journal.pone.0000869
Law AJ, Wang YH, Sei Y, O’Donnell P, Piantadosi P, Papaleo F, Straub RE, Huang WW et al (2012) Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110 delta inhibition as a potential therapeutic strategy. Proc Natl Acad Sci U S A 109(30):12165–12170. https://doi.org/10.1073/pnas.1206118109
Acknowledgements
The authors would like to thank Beijing LabWecome Technology Co., Ltd. for technical support.
Funding
This work study was partially supported by the Beijing Excellent Talent Training Funding (grant no. 2017000021469G215), the Natural Science Foundation of Capital Medical University of China (grant nos. PYZ2017082; PYZ2018081), the National Key Research and Development Program of China (grant no. 2018YFF0301103), and the High Level Public Health Technology Talent Construction Project (grant nos. Leading Talent-02-05).
Author information
Authors and Affiliations
Contributions
Duo Zhang and Baoge Liu conceived and designed the study. Duo Zhang and Xuanyu Chen participated the cell experiments. Duo Zhang, Wei Cui, Yuan Yuan integrated and analyzed the experimental data. Duo Zhang drafted the manuscript. Di Zhu, Jichao Zhu, Shuo Duan, and Chenxi Li contributed the statistical analysis. Baoge Liu and Duo Zhang confirmed the authenticity of all the raw data. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
The manuscript does not contain data from any individual person.
Consent to Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, D., Chen, X., Liu, B. et al. The Temporal and Spatial Changes of Autophagy and PI3K Isoforms in Different Neural Cells After Hypoxia/Reoxygenation Injury. Mol Neurobiol 60, 5366–5377 (2023). https://doi.org/10.1007/s12035-023-03421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03421-9