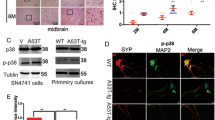
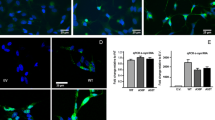
Abstract
Deletion and missense or nonsense mutation of RAB39B gene cause familial Parkinson’s disease (PD). We hypothesized that deletion and mutation of RAB39B gene induce degeneration of dopaminergic neurons by decreasing protein level of functional RAB39B and causing RAB39B deficiency. Cellular model of deletion or mutation of RAB39B gene-induced PD was prepared by knocking down endogenous RAB39B in human SH-SY5Y dopaminergic cells. Transfection of shRNA-induced 90% reduction in RAB39B level significantly decreased viability of SH-SY5Y dopaminergic neurons. Deficiency of RAB39B caused impairment of macroautophagy/autophagy, which led to increased protein levels of α-synuclein and phospho-α-synucleinSer129 within endoplasmic reticulum (ER) and mitochondria. RAB39B deficiency-induced increase of ER α-synuclein and phospho-α-synucleinSer129 caused activation of ER stress, unfolded protein response, and ER stress-induced pro-apoptotic cascade. Deficiency of RAB39B-induced increase of mitochondrial α-synuclein decreased mitochondrial membrane potential and increased mitochondrial superoxide. RAB39B deficiency-induced activation of ER stress pro-apoptotic pathway, mitochondrial dysfunction, and oxidative stress caused apoptotic death of SH-SY5Y dopaminergic cells by activating mitochondrial apoptotic cascade. In contrast to neuroprotective effect of wild-type RAB39B, PD mutant (T168K), (W186X), or (G192R) RAB39B did not prevent tunicamycin- or rotenone-induced increase of neurotoxic α-synuclein and activation of pro-apoptotic pathway. Our results suggest that RAB39B is required for survival and macroautophagy function of dopaminergic neurons and that deletion or PD mutation of RAB39B gene-induced RAB39B deficiency induces apoptotic death of dopaminergic neurons via impairing autophagy function and upregulating α-synuclein.
Graphical Abstract














Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nature Rev Mol Cell Biol 10:513–525
HommaY HS, Fukuda M (2021) Rab family of small GTPases: an updated view on their regulation and functions. FEBS J 288:36–55
Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D’Elia E et al (2010) Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet 86:185–195
Wilson GR, Sim JC, McLean C, Giannandrea M, Galea CA, Riseley JR et al (2014) Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with α-synuclein pathology. Am J Hum Genet 95:729–735
Mata IF, Jang Y, Kim CH, Hanna DS, Dorschner MO, Samii A et al (2015) The RAB39B p.G192R mutation causes X-linked dominant Parkinson’s disease. Mol Neurodegener 10:50
Lesage S, Bras J, Cormier-Dequaire F, Condroyer C, Nicolas A, Darwent L et al (2015) Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol Genet 1:e9
Güldner M, Schulte C, Hauser AK, Gasser T, Brockmann K (2016) Broad clinical phenotype in Parkinsonism associated with a base pair deletion in RAB39B and additional POLG variant. Parkinsonism Relat Disord 31:148–150
Ciammola A, Carrera P, Di Fonzo A, Sassone J, Villa R, Poletti B et al (2017) X-linked parkinsonism with intellectual disability caused by novel mutations and somatic mosaicism in RAB39B gene. Parkinsonism Relat Disord 44:142–146
Gao Y, Wilson GR, Stephenson SEM, Bozaoglu K, Lockhart FMJ, PJ, (2018) The emerging role of Rab GTPases in the pathogenesis of Parkinson’s disease. Mov Disorder 33:196–207
Tang BL (2021) RAB39B’s role in membrane traffic, autophagy, and associated neuropathology. J Cellular Physiol 236:1579–1592
Yua L, Chena Y, Tooze SA (2018) Autophagy pathway: cellular and molecular mechanisms. Autophagy 14:207–215
Melia TJ, Lystad AH, Simonsen A (2020) Autophagosome biogenesis: from membrane growth to closure. J Cell Biol 219:e202002085
Nakatogawa H (2020) Mechanisms governing autophagosome biogenesis. Nature Rev Mol Cell Biol 21:439–458
Chang C, Jensen LE, Hurley JH (2021) Autophagosome biogenesis comes out of the black box. Nature Cell Biol 23:450–456
Menzies FM, Fleming A, Rubinsztein DC (2015) Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 16:345–357
Bingol B (2018) Autophagy and lysosomal pathways in nervous system disorders. Mol Cell Neurosci 91:167–208
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884
Giovedì S, Ravanelli MM, Parisi B, Bettegazzi B, Guarnieri FC (2020) Dysfunctional autophagy and endolysosomal system in neurodegenerative diseases: relevance and therapeutic options. Front Cell Neurosci 14:602116
Park H, Kang JH, Lee S (2020) Autophagy in neurodegenerative diseases: a hunter for aggregates. Int J Mol Sci 21:3369
Arotcarena ML, Teil M, Dehay B (2019) Autophagy in synucleinopathy: the overwhelmed and defective machinery. Cells 8:565
Hou X, Watzlawik JO, Fiesel FC, Springer W (2020) Autophagy in Parkinson’s disease. J Mol Biol 432:2651–2672
Senkevich K, Gan-Or Z (2020) Autophagy lysosomal pathway dysfunction in Parkinson’s disease; evidence from human genetics. Parkinsonism Relat Disord 73:60–71
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72:257–268
Sellier C, Campanari ML, Corbier CJ, Gaucherot A, Kolb-Cheynel I, Oulad-Abdelghani M et al (2016) Loss of C9ORF72 impairs autophagy and synergizes with polyQ ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J 35:1276–1297
Sullivan PM, Zhou X, Robins AM, Paushter DH, Kim D, Smolka MB et al (2016) The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta Neuropathol Commun 4:51
Yang M, Liang C, Swaminathan K, Herrlinger S, Lai F, Shiekhattar R et al (2016) C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci Adv 2:e1601167
Webster CP, Smith FF, Bauer CS, Moller A, Hautbergue GM, Ferraiuolo L et al (2016) The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J 35:1656–1676
Beckers J, Tharkeshwar AK, Van Damme P (2021) C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels. Autophagy 17:3306–3322
Wong YC, Krainc D (2017) α-Synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nature Med 23:1–13
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27:27–42
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J et al (2003) Alpha-synuclein locus triplication causes Parkinson’s disease. Science 302:841–845
Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S et al (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N et al (2002) Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci 22:2780–2791
Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP et al (2008) The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci USA 105:763–768
Han D, Zheng W, Wang X, Chen Z (2020) Proteostasis of α-synuclein and its role in the pathogenesis of Parkinson’s disease. Front Cell Neurosci 14:45
Serratos IN, Hernández-Pérez E, Campos C, Aschner M, Santamaría A (2022) An update on the critical role of α-synuclein in Parkinson’s disease and other synucleinopathies: from tissue to cellular and molecular levels. Mol Neurobiol 59:620–642
Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC (2003) Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013
Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283:23542–23556
Ahmed I, Liang Y, Schools S, Dawson VL, Dawson TM, Savitt JM (2012) Development and characterization of a new Parkinson’s disease model resulting from impaired autophagy. J Neurosci 32:16503–16509
Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B et al (2019) Endoplasmic reticulum stress signaling- from basic mechanisms to clinical applications. FEBS J 286:241–278
Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nature Rev Mol Cell Biol 21:421–436
Iurlaro R, Muñoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283:2640–2652
Mercado G, Valdés P, Hetz C (2013) An ERcentric view of Parkinson’s disease. Trends Mol Med 19:165–175
Colla E (2019) Linking the endoplasmic reticulum to Parkinson’s disease and alpha-synucleinopathy. Front Neurosci 13:560
da Costa CA, El Manaa W, Duplan E, Checler F (2020) The endoplasmic reticulum stress/unfolded protein response and their contributions to Parkinson’s disease physiopathology. Cells 9:2495
Hoozemans JJM, van Haastert ES, Eikelenboom P, de Vos RAI, Rozemuller JM, Scheper W (2007) Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun 354:707–711
Mercado G, Castillo V, Soto P, López N, Axten JM, Sardi SP et al (2018) Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson’s disease. Neurobiol Dis 112:132–148
Coppola-Segovia V, Cavarsan C, Maia FG, Ferraz AC, Nakao LS, Lima M et al (2017) ER stress induced by tunicamycin triggers α-synuclein oligomerization, dopaminergic neurons death and locomotor impairment: a new model of Parkinson’s disease. Mol Neurobiol 54:5798–5806
Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ et al (2006) Phosphorylation of ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281:29739–29752
Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK (2012) Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J Neurosci 32:3301–3305
Sugeno N, Takeda A, Hasegawa T, Kobayashi M, Kikuchi A, Mori F et al (2008) Serine 129 phosphorylation of alpha-synuclein induces unfolded protein response-mediated cell death. J Biol Chem 283:23179–23188
Heman-Ackah SM, Manzano R, Hoozemans JJM, Scheper W, Flynn R, Haerty W et al (2017) Alpha-synuclein induces the unfolded protein response in Parkinson’s disease SNCA triplication iPSC-derived neurons. Hum Mol Genet 26:4441–4450
Gorbatyuk MS, Shabashvili A, Chen W, Meyers C, Sullivan LF, Salganik M et al (2012) Glucose regulated protein 78 diminishes α-synuclein neurotoxicity in a rat model of Parkinson disease. Mol Therapy 20:1327–1337
Bose A, Beal MF (2016) Mitochondrial dysfunction in Parkinson’s disease. J Neurochem 139:216–231
Trist BG, Hare DJ, Double KL (2019) Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 18:e13031
Borsche M, Pereira SL, Klein C, Grunewald A (2021) Mitochondria and Parkinson’s disease: clinical, molecular, and translational aspects. J Parkinson’s Disease 11:45–60
Trinh D, Israwi AR, Arathoon LR, Gleave JA, Nash JE (2021) The multi-faceted role of mitochondria in the pathology of Parkinson’s disease. J Neurochem 156:715–752
Vicario M, Cieri D, Brini M, Calì T (2018) The close encounter between alpha-synuclein and mitochondria. Frontiers in Neurosci 12:388
Haque ME, Akther M, Azam S, Kim IS, Lin Y, Lee YH et al (2022) Targeting α-synuclein aggregation and its role in mitochondrial dysfunction in Parkinson’s disease. Br J Pharmacol 179:23–45
Mak SK, Tewari D, Tetrud JW, Langston JW, Schüle B (2011) Mitochondrial dysfunction in skin fibroblasts from a Parkinson’s disease patient with an alpha-synuclein triplication. J Parkinson’s Disease 1:175–183
Ordonez DG, Lee MK, Feany MB (2018) α-Synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97:108–124
Gao G, Wang Z, Lu L, Duan C, Wang X, Yang H (2017) Morphological analysis of mitochondria for evaluating the toxicity of α-synuclein in transgenic mice and isolated preparations by atomic force microscopy. Biomed Pharmacotherapy 96:1380–1388
McMillan CR, Sharma R, Ottenhof T, Niles LP (2007) Modulation of tyrosine hydroxylase expression by melatonin in human SH-SY5Y neuroblastoma cells. Neurosci Lett 419:202–206
Ganapathy K, Indrani D, Sowmithra S, Joshi P, Bhonde R (2016) Influence of 6-hydroxydopamine toxicity on α-synuclein phosphorylation, resting vesicle expression, and vesicular dopamine release. J Cell Biochem 117:2719–2736
Xicoy H, Wieringa B, Martens GJM (2017) The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol Neurodegener 12:10
Chiu CC, Lu CS, Weng YH, Chen YL, Huang YZ, Chen RS et al (2019) PARK14 (D331Y) PLA2G6 causes early-onset degeneration of substantia nigra dopaminergic neurons by inducing mitochondrial dysfunction, ER stress, mitophagy impairment and transcriptional dysregulation in a knockin mouse model. Mol Neurobiol 56:3835–3853
Uberti VH, de Freitas BS, Molz P, Bromberg E, Schröder N (2020) Iron overload impairs autophagy: effects of rapamycin in ameliorating iron-related memory deficits. Mol Neurobiol 57:1044–1054
Li J, Tian M, Hua T, Wang HW, Yang M, Li WQ et al (2021) Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy 17:4062–4082
Liebl MP, Meister SC, Frey L, Hendrich K, Klemmer A, Wohlfart B et al (2022) Robust LC3B lipidation analysis by precisely adjusting autophagic flux. Sci Rep 12:79
Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A et al (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin induced cell death. J Cell Biol 171:603–614
Esposito A, Falace A, Wagner M, Gal M, Mei D, Conti V et al (2019) Biallelic DMXL2 mutations impair autophagy and cause Ohtahara syndrome with progressive course. Brain 142:3876–3891
Seto S, Sugaya K, Tsujimura K, Nagata T, Horii T, Koide Y (2013) Rab39a interacts with phosphatidylinositol 3-kinase and negatively regulates autophagy induced by lipopolysaccharide stimulation in macrophages. PLoS ONE 8:e83324
Minamino T, Kitakaze M (2010) ER stress in cardiovascular disease. J Mol Cell Cardiol 48:1105–1110
Zheng JH, Follis AV, Kriwacki RW, Moldoveanu T (2016) Discoveries and controversies in BCL-2 protein-mediated apoptosis. FEBS J 283:2690–2700
Mou Z, Yuan YH, Lou YX, Heng Y, Huang JY, Xia CY et al (2016) Bibenzyl compound 20c protects against endoplasmic reticulum stress in tunicamycin-treated PC12 cells in vitro. Acta Pharmacol Sin 37:1525–1533
Chiu CC, Yeh TH, Lu CS, Huang YC, Cheng YC, Huang YZ et al (2017) PARK14 PLA2G6 mutants are defective in preventing rotenone-induced mitochondrial dysfunction, ROS generation and activation of mitochondrial apoptotic pathway. Oncotarget 8:79046–79060
da Silva DC, Valentão P, Andrade PB, Pereira DM (2020) Endoplasmic reticulum stress signaling in cancer and neurodegenerative disorders: Tools and strategies to understand its complexity. Pharmacol Res 155:104702
Liu X, Qu L, Zhang N, Yu X, Xiao Z, Song L et al (2021) Ndfip1 prevents rotenone-induced neurotoxicity and upregulation of α-synuclein in SH-SY5Y cells. Front Mol Neurosci 13:613404
Zavodszky E, Seaman MNJ, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME et al (2014) Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nature Commun 5:3828
Quinn PMJ, Moreira PI, Francisco Ambrósio A, Alves CH (2020) PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol Commun 8:189
Malpartida AB, Williamson M, Narendra DP, Wade-Martins R, Ryan BJ (2021) Mitochondrial dysfunction and mitophagy in Parkinson’s disease: from mechanism to therapy. Trends Biochem Sci 46:329–343
Boecker CA, Holzbaur ELF (2021) Hyperactive LRRK2 kinase impairs the trafficking of axonal autophagosomes. Autophagy 17:2043–2045
Wauters F, Cornelissen T, Imberechts D, Martin S, Koentjoro B, Sue C et al (2020) LRRK2 mutations impair depolarization-induced mitophagy through inhibition of mitochondrial accumulation of RAB10. Autophagy 16:203–222
Gao Y, Martínez-Cerdeño V, Hogan KJ, McLean CA, Lockhart PJ (2020) Clinical and neuropathological features associated with loss of RAB39B. Mov Disorder 35:687–693
Ho DH, Kim H, Nam D, Sim H, Kim J, Kim HG et al (2018) LRRK2 impairs autophagy by mediating phosphorylation of leucyl-tRNA synthetase. Cell Biochem Funct 36:431–442
Lee JH, Han JH, Kim H, Park SM, Joe EH, Jou I (2019) Parkinson’s disease-associated LRRK2-G2019S mutant acts through regulation of SERCA activity to control ER stress in astrocytes. Acta Neuropathol Commun 7:68
Yokota T, Sugawara K, Ito K, Takahashi R, Ariga H, Mizusawa H (2003) Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochem Biophys Res Commun 312:1342–1348
Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J et al (2011) Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ 18:769–782
Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X (2012) Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem 121:830–839
Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S et al (2014) PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science 344:203–207
Chiu CC, Weng YH, Huang YZ, Chen RS, Liu YC, Yeh TH et al (2020) (D620N) VPS35 causes the impairment of Wnt/β-catenin signaling cascade and mitochondrial dysfunction in a PARK17 knockin mouse model. Cell Death Dis 11:1018
Singh A, Zhi L, Zhang H (2019) LRRK2 and mitochondria: recent advances and current views. Brain Res 1702:96–104
Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91:119–149
Pylypenko O, Hammich H, Yu IM, Houdusse A (2018) Rab GTPases and their interacting protein partners: structural insights into Rab functional diversity. Small GTPases 9:22–48
Funding
This work was supported by Chang Gung Medical Foundation (CMRPD1H0282, CMRPD1H0283, and CMRPD1M0141 to HL Wang; CMRPG3K0952, CMRPG3J0763, and CMRPD1M0291 to CC Chiu), the Ministry of Science and Technology, Taiwan (MOST 110–2320-B-182–003-MY3 to HL Wang; MOST109-2314-B-182–081-MY3 and MOST111-2326-B-182–001-MY3 to CC Chiu), and Healthy Aging Research Center, Chang Gung University (EMRPD1M0451 to HL Wang and UMRPD1M0341 to CC Chiu).
Author information
Authors and Affiliations
Contributions
Ching-Chi Chiu, Yi-Hsin Weng, Tu-Hsueh Yeh, and Hung-Li Wang conceptualized the study and designed the experiments; Ching-Chi Chiu, Yi-Hsin Weng, Juu-Chin Lu, Wan-Shia Chen, Allen Han-Ren Li, and Ying-Ling Chen performed the experiments and analyzed the data; Ching-Chi Chiu, Yi-Hsin Weng, Kuo-Chen Wei, and Hung-Li Wang wrote the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable. This is an observational study. The Chang Gung University Research Ethics Committee has confirmed that no ethical approval is required.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiu, CC., Weng, YH., Yeh, TH. et al. Deficiency of RAB39B Activates ER Stress-Induced Pro-apoptotic Pathway and Causes Mitochondrial Dysfunction and Oxidative Stress in Dopaminergic Neurons by Impairing Autophagy and Upregulating α-Synuclein. Mol Neurobiol 60, 2706–2728 (2023). https://doi.org/10.1007/s12035-023-03238-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03238-6