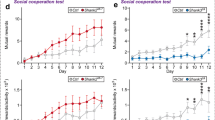
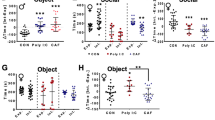
Abstract
Autism spectrum disorder (ASD) represents a heterogeneous group of neurodevelopmental disorders characterized by deficits in social communication, social interaction, and the presence of restricted repetitive behaviors. The cause of ASD involves complex interactions between genetic and environmental factors. Haploinsufficiency of the Coiled-coil and C2 domain containing 1A (Cc2d1a) gene is causally linked to ASD, and obesity has been associated with worse outcomes for ASD. High-fat diet (HFD) feeding leads to the development of obesity and metabolic dysfunction; however, the effect of HFD on pre-existing autistic-like phenotypes remains to be clarified. Here, we report that male Cc2d1a conditional knockout (cKO) mice fed with HFD, from weaning onwards and throughout the experimental period, show a marked aggravation in autistic-like phenotypes, manifested in increased restricted repetitive behaviors and impaired performance in the preference for social novelty, but not in sociability and cognitive impairments assessed using the object location memory, novel object recognition, and Morris water maze tests. HFD feeding also results in increased numbers of reactive microglia and astrocytes, and exacerbates reductions in dendritic complexity and spine density of hippocampal CA1 pyramidal neurons. Furthermore, we demonstrate that chronic treatment with minocycline, a semisynthetic tetracycline-derived antibiotic, rescues the observed behavioral and morphological deficits in Cc2d1a cKO mice fed with HFD. Collectively, these findings highlight an aggravating role of HFD in pre-existing autistic-like phenotypes and suggest that minocycline treatment can alleviate abnormal neuronal morphology and behavioral symptoms associated with ASD resulted from the interplay between genetic and environmental risk factors.
Graphical Abstract











Similar content being viewed by others
Data Availability
The authors confirm that all data generated and analyzed during this study are either included in this published article or available from the corresponding authors upon reasonable request.
Abbreviations
- ANOVA :
-
Analysis of variance
- ASD :
-
Autism spectrum disorder
- BTBR :
-
BTBR T+ Itpr3tf/J
- CA1 :
-
Cornu ammonis 1
- Cc2d1a :
-
Coiled-coil and C2 domain containing 1A
- Cc2d1a f / f :
-
Cc2d1a Floxed
- cKO:
-
Conditional knockout
- Env :
-
Environmental
- Gen :
-
Genetic
- GFAP :
-
Glial fibrillary acidic protein
- HFD :
-
High-fat diet
- MWM :
-
Morris water maze
- Iba1 :
-
Ionized calcium-binding adapter molecule-1
- ND :
-
Normal diet
- NOR :
-
Novel object recognition
- OLM :
-
Object location memory
- Mino :
-
Minocycline
- P0 :
-
Postnatal day 0
- PBS :
-
Phosphate-buffered saline
- PFA :
-
Paraformaldehyde
- RM :
-
Repeated measured
- WT :
-
Wild-type
References
Baron-Cohen S, Belmonte MK (2005) Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci 28:109–126. https://doi.org/10.1146/annurev.neuro.27.070203.144137
Chen JA, Peñagarikano O, Belgard TG, Swarup V, Geschwind DH (2015) The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol 10:111–144. https://doi.org/10.1146/annurev-pathol-012414-040405
Heavner WE, Smith SEP (2020) Resolving the synaptic versus developmental dichotomy of autism risk genes. Trends Neurosci 43(4):227–241. https://doi.org/10.1016/j.tins.2020.01.009
Satterstrom FK, Kosmicki JA, Wang J, Breen MS, Rubeis SD, An JY et al (2020) Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of Autism. Cell 180(3):568–584. https://doi.org/10.1016/j.cell.2019.12.036
Miles JH (2011) Autism spectrum disorders-a genetics review. Genet Med 13(4):278–294. https://doi.org/10.1097/GIM.0b013e3181ff67ba
Yoo H (2015) Genetics of autism spectrum disorder: current status and possible clinical applications. Exp Neurobiol 24(4):257–272. https://doi.org/10.5607/en.2015.24.4.257
Kim YS, Leventhal BL (2015) Genetic epidemiology and insights into interactive genetic and environmental effects in autism spectrum disorders. Biol Psychiatry 77(1):66–74. https://doi.org/10.1016/j.biopsych.2014.11.001
Fuccillo MV (2016) Striatal circuits as a common node for Autism pathophysiology. Front Neurosci 10:27. https://doi.org/10.3389/fnins.2016.00027
Bölte S, Girdler S, Marschik PB (2019) The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci 76(7):1275–1297. https://doi.org/10.1007/s00018-018-2988-4
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T et al (2011) Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68(11):1095–1102. https://doi.org/10.1001/archgenpsychiatry.2011.76
Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A (2014) The familial risk of autism. JAMA 311(17):1770–1777. https://doi.org/10.1001/jama.2014.4144
Modabbernia A, Velthorst E, Reichenberg A (2017) Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism 8:13. https://doi.org/10.1186/s13229-017-0121-4
Emberti Gialloreti L, Mazzone L, Benvenuto A, Fasano A, Alcon AG, Kraneveld A et al (2019) Risk and protective environmental factors associated with autism spectrum disorder: evidence-based principles and recommendations. J Clin Med 8(2):217. https://doi.org/10.3390/jcm8020217
Silverman JL, Yang M, Lord C, Crawley JN (2010) Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11(7):490–502. https://doi.org/10.1038/nrn2851
Banerjee S, Riordan M, Bhat MA (2014) Genetic aspects of autism spectrum disorders: insights from animal models. Front Cell Neurosci 8:58. https://doi.org/10.3389/fncel.2014.00058
Millar AM, Souslova T, Albert PR (2012) The Freud-1/CC2D1A family: multifunctional regulators implicated in mental retardation. In: Tan PU, editor. Latest findings in intellectual and developmental disabilities research. InTech; 51000 Rijeka, Croatia: pp. 279–302.
Manzini MC, Xiong L, Shaheen R, Tambunan DE, Di Costanzo S, Mitisalis V et al (2014) CC2D1A regulates human intellectual and social function as well as NF-κB signaling homeostasis. Cell Rep 8(3):647–655. https://doi.org/10.1016/j.celrep.2014.06.039
Oaks AW, Zamarbide M, Tambunan DE, Santini E, Di Costanzo S, Pond HL et al (2017) Cc2d1a loss of function disrupts functional and morphological development in forebrain neurons leading to cognitive and social deficits. Cereb Cortex 27(2):1670–1685. https://doi.org/10.1093/cercor/bhw009
Yang CY, Yu TH, Wen WL, Ling P, Hsu KS (2019) Conditional deletion of CC2D1A reduces hippocampal synaptic plasticity impairs cognitive function through Rac1 hyperactivation. J Neurosci 39(25):4959–4975. https://doi.org/10.1523/JNEUROSCI.2395-18.2019
Yang CY, Hung YC, Cheng KH, Ling P, Hsu KS (2021) Loss of CC2D1A in glutamatergic neurons results in autistic-like features in mice. Neurotherapeutics 18(3):2021–2039. https://doi.org/10.1007/s13311-021-01072-z
Broder-Fingert S, Brazauskas K, Lindgren K, Iannuzzi D, Van Cleave J (2014) Prevalence of overweight and obesity in a large clinical sample of children with autism. Acad Pediatr 14(4):408–414. https://doi.org/10.1016/j.acap.2014.04.004
Hill AP, Zuckerman KE, Fombonne E (2015) Obesity and Autism. Pediatrics 136(6):1051–1061. https://doi.org/10.1542/peds.2015-1437
Zheng Z, Zhang L, Li S, Zhao F, Wang Y, Huang L et al (2017) Association among obesity, overweight and autism spectrum disorder: a systematic review and meta-analysis. Sci Rep 7(1):11697. https://doi.org/10.1038/s41598-017-12003-4
Kahathuduwa CN, West BD, Blume J, Dharavath N, Moustaid-Moussa N, Mastergeorge A (2019) The risk of overweight and obesity in children with autism spectrum disorders: a systematic review and meta-analysis. Obes Rev 20(12):1667–1679. https://doi.org/10.1111/obr.12933
Zilkha N, Kuperman Y, Kimchi T (2017) High-fat diet exacerbates cognitive rigidity and social deficiency in the BTBR mouse model of autism. Neuroscience 345:142–154. https://doi.org/10.1016/j.neuroscience.2016.01.070
Zamarbide M, Mossa A, Muñoz-Llancao P, Wilkinson MK, Pond HL, Oaks AW et al (2019) Male-specific cAMP signaling in the hippocampus controls spatial memory deficits in a mouse model of Autism and intellectual disability. Biol Psychiatry 85(9):760–768. https://doi.org/10.1016/j.biopsych.2018.12.013
Mossa A, Manzini MC (2021) Molecular causes of sex-specific deficits in rodent models of neurodevelopmental disorders. J Neurosci Res 99(1):37–56. https://doi.org/10.1002/jnr.24577
Deacon RM (2006) Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 1(3):122–124. https://doi.org/10.1038/nprot.2006.170
Karvat G, Kimchi T (2012) Systematic autistic-like behavioral phenotyping of 4 mouse strains using a novel wheel-running assay. Behav Brain Res 233(2):405–414. https://doi.org/10.1016/j.bbr.2012.05.028
Lin YT, Hsieh TY, Tsai TC, Chen CC, Huang CC, Hsu KS (2018) Conditional deletion of hippocampal CA2/CA3a oxytocin receptors impairs the persistence of long-term social recognition memory in mice. J Neurosci 38(5):1218–1231. https://doi.org/10.1523/JNEUROSCI.1896-17.2017
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory.’ Nat Protoc 1(3):1306–1311. https://doi.org/10.1038/nprot.2006.205
Dong Z, Bai Y, Wu X, Li H, Gong B, Howland JG et al (2013) Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology 64:65–73. https://doi.org/10.1016/j.neuropharm.2012.06.027
Tyler WJ, Pozzo-Miller L (2003) Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurons. J Physiol 553(2):497–509. https://doi.org/10.1113/jphysiol.2003.052639
Dansie LE, Phommahaxay K, Okusanya AG, Uwadia J, Huang M, Rotschafer SE et al (2013) Long-lasting effects of minocycline on behavior in young but not adult Fragile X mice. Neuroscience 246:186–198. https://doi.org/10.1016/j.neuroscience.2013.04.058
Troyb E, Knoch K, Herlihy L, Stevens MC, Chen CM, Barton M et al (2016) Restricted and repetitive behaviors as predictors of outcome in autism spectrum disorders. J Autism Dev Disord 46(4):1282–1296. https://doi.org/10.1007/s10803-015-2668-2
Baufeld C, Osterloh A, Prokop S, Miller KR, Heppner FL (2016) High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol 132(3):361–375. https://doi.org/10.1007/s00401-016-1595-4
Nakandakari SCBR, Muñoz VR, Kuga GK, Gaspar RC, Sant’Ana MR, Pavan ICB, et al (2019) Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav Immun 79:284–293. https://doi.org/10.1016/j.bbi.2019.02.016
Cunningham CL, Martínez-Cerdeño V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33(10):4216–4233. https://doi.org/10.1523/JNEUROSCI.3441-12.2013
Emadi-Kouchak H, Mohammadinejad P, Asadollahi-Amin A, Rasoulinejad M, Zeinoddini A, Yalda A et al (2016) Therapeutic effects of minocycline on mild-to-moderate depression in HIV patients: a double-blind, placebo-controlled, randomized trial. Int Clin Psychopharmacol 31(1):20–26. https://doi.org/10.1097/YIC.0000000000000098
Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M et al (2018) Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 8(1):27. https://doi.org/10.1038/s41398-017-0073-7
Zhang L, Zheng H, Wu R, Zhu F, Kosten TR, Zhang XY et al (2018) Minocycline adjunctive treatment to risperidone for negative symptoms in schizophrenia: association with pro-inflammatory cytokine levels. Prog Neuropsychopharmacol Biol Psychiatry 85:69–76. https://doi.org/10.1016/j.pnpbp.2018.04.004
Chini M, Pöpplau JA, Lindemann C, Carol-Perdiguer L, Hnida M, Oberländer V et al (2020) Resolving and rescuing developmental miswiring in a mouse model of cognitive impairment. Neuron 105(1):60–74. https://doi.org/10.1016/j.neuron.2019.09.042
Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K et al (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4(3):e525. https://doi.org/10.1038/cddis.2013.54
Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB et al (2019) Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci 22(3):374–385. https://doi.org/10.1038/s41593-018-0334-7
Tsuang MT, Bar JL, Stone WS, Faraone SV (2004) Gene-environment interactions in mental disorders. World Psychiatry 3:73–83
Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL et al (2016) Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. J Autism Dev Disord 46(5):3729–3738. https://doi.org/10.1542/peds.2011-2583
Reynolds LC, Inder TE, Neil JJ, Pineda RG, Rogers CE (2014) Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J Perinatol 34(9):688–692. https://doi.org/10.1038/jp.2014.80
Sullivan EL, Riper KM, Lockard R, Valleau JC (2015) Maternal high-fat diet programming of the neuroendocrine system and behavior. Horm Behav 76:153–161. https://doi.org/10.1016/j.yhbeh.2015.04.008
Hariri N, Thibault L (2010) High-fat diet-induced obesity in animal models. Nutr Res Rev 23(2):270–299. https://doi.org/10.1017/S0954422410000168
James AM, Collins Y, Logan A, Murphy MP (2012) Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol Metab 23(9):429–434. https://doi.org/10.1016/j.tem.2012.06.008
Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E 4th, Taylor CM, Welsh DA et al (2015) Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77(7):607–615. https://doi.org/10.1016/j.biopsych.2014.07.012
Takase K, Tsuneoka Y, Oda S, Kuroda M, Funato H (2016) High-fat diet feeding alters olfactory-, social-, and reward-related behaviors of mice independent of obesity. Obesity (Silver Spring) 24(4):886–894. https://doi.org/10.1002/oby.21441
Deng W, Li F, Ke H, Wang S, Li Z, Lv P et al (2022) Effect of metformin in autistic BTBR T + Itpr3tf/J mice administered a high-fat diet. Brain Res Bull 183:172–183. https://doi.org/10.1016/j.brainresbull.2022.02.021
Wang Z, Fan J, Wang J, Li Y, Xiao L, Duan D et al (2016) Protective effect of lycopene on high-fat diet-induced cognitive impairment in rats. Neurosci Lett 627:185–191. https://doi.org/10.1016/j.neulet.2016.05.014
Dalvi PS, Chalmers JA, Luo V, Han DY, Wellhauser L, Liu Y et al (2017) High fat induces acute and chronic inflammation in the hypothalamus: effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int J Obes (Lond) 41(1):149–158. https://doi.org/10.1038/ijo.2016.183
Zou C, Shi Y, Ohli J, Schüller U, Dorostkar MM, Herms J (2016) Neuroinflammation impairs adaptive structural plasticity of dendritic spines in a preclinical model of Alzheimer’s disease. Acta Neuropathol 131(2):235–246. https://doi.org/10.1007/s00401-015-1527-8
Moser MB, Trommald M, Egeland T, Andersen P (1997) Spatial training in a complex environment and isolation alter the spine distribution differently in rat CA1 pyramidal cells. J Comp Neurol 380(3):373–381. https://doi.org/10.1002/(sici)1096-9861(19970414)380:3%3c373::aid-cne6%3e3.0.co;2-#
Pereira AC, Lambert HK, Grossman YS, Dumitriu D, Waldman R, Jannetty SK et al (2014) Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci USA 111(52):18733–18738. https://doi.org/10.1073/pnas.1421285111
Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR (2013) Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex 23(8):1784–1797. https://doi.org/10.1093/cercor/bhs151
Garwood CJ, Cooper JD, Hanger DP, Noble W (2010) Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front Psychiatry 1:136. https://doi.org/10.3389/fpsyt.2010.00136
Pardo CA, Buckley A, Thurm A, Lee LC, Azhagiri A, Neville DM et al (2013) A pilot open-label trial of minocycline in patients with autism and regressive features. J Neurodev Disord 5(1):9. https://doi.org/10.1186/1866-1955-5-9
Garrido-Mesa N, Zarzuelo A, Gálvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169(2):337–352. https://doi.org/10.1111/bph.12139
Vaughn AC, Cooper EM, DiLorenzo PM, O’Loughlin LJ, Konkel ME, Peters JH et al (2017) Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol Exp (Wars) 77(1):18–30. https://doi.org/10.21307/ane-2017-033
Eshraghi AA, Liu G, Kay SS, Eshraghi RS, Mittal J, Moshiree B et al (2018) Epigenetics and autism spectrum disorder: is there a correlation? Front Cell Neurosci 12:78. https://doi.org/10.3389/fncel.2018.00078
Wilkinson B, Campbell DB (2013) Contribution of long noncoding RNAs to autism spectrum disorder risk. Int Rev Neurobiol 113:35–59. https://doi.org/10.1016/B978-0-12-418700-9.00002-2
Wang J, Wang L (2020) Prediction and prioritization of autism-associated long non-coding RNAs using gene expression and sequence features. BMC Bioinformatics 21(1):505. https://doi.org/10.1186/s12859-020-03843-5
Abedpoor N, Taghian F, Hajibabaie F (2022) Cross brain-gut analysis highlighted hub genes and LncRNA networks differentially modified during leucine consumption and endurance exercise in mice with depression-like behaviors. Mol Neurobio 59(7):4106–4123. https://doi.org/10.1007/s12035-022-02835-1
Acknowledgements
The authors thank the technical services provided by the Bio-image Core Facility of the National Core Facility Program for Biotechnology, Ministry of Science and Technology, Taiwan.
Funding
This work was supported by research grants from the National Health Research Institute (NHRI-EX109-10912NI; NHRI-EX110-10912NI) and the Ministry of Science and Technology (109–2320-B-006–039-MY3 and 110–2320-B-006–036-MY3), Taiwan.
Author information
Authors and Affiliations
Contributions
YCW, CHC, CYY, and KSH designed research; YCW, CHC, and CYY performed research; YCW, CHC, and CYY analyzed data; PL provided Cc2d1af/f mice; YCW, CHC, and KSH wrote the paper.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the National Cheng Kung University (authorization #110077).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, YC., Chen, CH., Yang, CY. et al. High-Fat Diet Exacerbates Autistic-Like Restricted Repetitive Behaviors and Social Abnormalities in CC2D1A Conditional Knockout Mice. Mol Neurobiol 60, 1331–1352 (2023). https://doi.org/10.1007/s12035-022-03146-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03146-1