Abstract
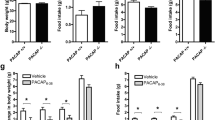
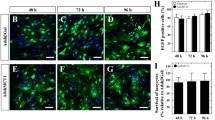
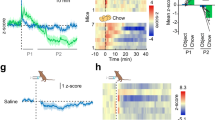
We have previously shown that pituitary adenylate cyclase-activating polypeptide (PACAP) in the ventromedial hypothalamus (VMH) enhances feeding during the dark cycle and after fasting, and inhibits feeding during the light cycle. On the other hand, galanin is highly expressed in the hypothalamus and has been reported to be involved in feeding regulation. In this study, we investigated the involvement of the VMH-PACAP to the dorsomedial hypothalamus (DMH)-galanin signaling in the regulation of feeding. Galanin expression in the hypothalamus was significantly increased with fasting, but this increment was canceled in PACAP-knockout (KO) mice. Furthermore, overexpression of PACAP in the VMH increased the expression of galanin, while knockdown (KD) of PACAP in the VMH decreased the expression of galanin, indicating that the expression of galanin in the hypothalamus might be regulated by PACAP in the VMH. Therefore, we expressed the synaptophysin-EGFP fusion protein (SypEGFP) in PACAP neurons in the VMH and visualized the neural projection to the hypothalamic region where galanin was highly expressed. A strong synaptophysin-EGFP signal was observed in the DMH, indicating that PACAP-expressing cells of the VMH projected to the DMH. Furthermore, galanin immunostaining in the DMH showed that galanin expression was weak in PACAP-KO mice. When galanin in the DMH was knocked down, food intake during the dark cycle and after fasting was decreased, and food intake during the light cycle was increased, as in PACAP-KO mice. These results indicated that galanin in the DMH may regulate the feeding downstream of PACAP in the VMH.






Similar content being viewed by others
Data Availability
All data generated during this study are available upon reasonable request from the corresponding authors.
Abbreviations
- AAV 2/9:
-
AAV vector serotype 9
- AgRP:
-
Agouti-related peptide
- ANOVA:
-
Analysis of variance
- ARC:
-
Arcuate nucleus
- cDNA:
-
Complementary DNA
- DMH:
-
Dorsomedial hypothalamus
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- KD:
-
Knockdown
- KO:
-
Knockout
- MCH:
-
Melanin-containing hormone
- PACAP:
-
Pituitary adenylate cyclase-activating polypeptide
- POMC:
-
Pro-opiomelanocortin
- PVH:
-
Paraventricular hypothalamus
- qPCR:
-
Quantitative polymerase chain reaction
- RT:
-
Reverse transcription
- SypEGFP:
-
Synaptophysin-EGFP fusion protein
- VMH:
-
Ventromedial hypothalamus
- WT:
-
Wild-type
References
Muller TD, Bluher M, Tschop MH, DiMarchi RD (2021) Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov. https://doi.org/10.1038/s41573-021-00337-8
Nguyen TT, Kambe Y, Kurihara T, Nakamachi T, Shintani N, Hashimoto H, Miyata A (2020) Pituitary adenylate cyclase-activating polypeptide in the ventromedial hypothalamus Is responsible for food intake behavior by modulating the expression of agouti-related peptide in mice. Mol Neurobiol 57(4):2101–2114. https://doi.org/10.1007/s12035-019-01864-7
Wang R, Yuan J, Zhang C, Wang L, Liu Y, Song L, Zhong W, Chen X et al (2018) Neuropeptide Y-positive neurons in the dorsomedial hypothalamus are involved in the anorexic effect of angptl8. Front Mol Neurosci 11:451. https://doi.org/10.3389/fnmol.2018.00451
Jeong JH, Lee DK, Jo YH (2017) Cholinergic neurons in the dorsomedial hypothalamus regulate food intake. Mol Metab 6(3):306–312. https://doi.org/10.1016/j.molmet.2017.01.001
Liao GY, Kinney CE, An JJ, Xu B (2019) TrkB-expressing neurons in the dorsomedial hypothalamus are necessary and sufficient to suppress homeostatic feeding. Proc Natl Acad Sci U S A 116(8):3256–3261. https://doi.org/10.1073/pnas.1815744116
Imoto D, Yamamoto I, Matsunaga H, Yonekura T, Lee ML, Kato KX, Yamasaki T, Xu S et al (2021) Refeeding activates neurons in the dorsomedial hypothalamus to inhibit food intake and promote positive valence. Mol Metab 54:101366. https://doi.org/10.1016/j.molmet.2021.101366
Rust VA, Crosby KM (2021) Cholecystokinin acts in the dorsomedial hypothalamus of young male rats to suppress appetite in a nitric oxide-dependent manner. Neurosci Lett 764:136295. https://doi.org/10.1016/j.neulet.2021.136295
Faber CL, Deem JD, Phan BA, Doan TP, Ogimoto K, Mirzadeh Z, Schwartz MW, Morton GJ (2021) Leptin receptor neurons in the dorsomedial hypothalamus regulate diurnal patterns of feeding, locomotion, and metabolism. Elife 10:e63671. https://doi.org/10.7554/eLife.63671
Parker JA, Bloom SR (2012) Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 63(1):18–30. https://doi.org/10.1016/j.neuropharm.2012.02.004
Fang P, Yu M, Guo L, Bo P, Zhang Z, Shi M (2012) Galanin and its receptors: a novel strategy for appetite control and obesity therapy. Peptides 36(2):331–339. https://doi.org/10.1016/j.peptides.2012.05.016
Marcos P, Covenas R (2021) Neuropeptidergic control of feeding: focus on the galanin family of peptides. Int J Mol Sci 22(5):2544. https://doi.org/10.3390/ijms22052544
Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF (1994) Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci U S A 91(22):10375–10379. https://doi.org/10.1073/pnas.91.22.10375
Barson JR, Morganstern I, Leibowitz SF (2010) Galanin and consummatory behavior: special relationship with dietary fat, alcohol and circulating lipids. Exp Suppl 102:87–111. https://doi.org/10.1007/978-3-0346-0228-0_8
Cheung CC, Hohmann JG, Clifton DK, Steiner RA (2001) Distribution of galanin messenger RNA-expressing cells in murine brain and their regulation by leptin in regions of the hypothalamus. Neuroscience 103(2):423–432. https://doi.org/10.1016/s0306-4522(01)00012-4
Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, Miyazaki J et al (2001) Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci U S A 98(23):13355–13360. https://doi.org/10.1073/pnas.231094498
Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M et al (2014) Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits 8:76. https://doi.org/10.3389/fncir.2014.00076
Kambe Y, Thi TN, Hashiguchi K, Sameshima Y, Yamashita A, Kurihara T, Miyata A (2022) The dorsal hippocampal protein targeting to glycogen maintains ionotropic glutamate receptor subunits expression and contributes to working and short-term memories in mice. J Pharmacol Sci 148(1):108–115. https://doi.org/10.1016/j.jphs.2021.10.008
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307(5712):1098–1101. https://doi.org/10.1126/science.1106148
Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C et al (2014) A mesoscale connectome of the mouse brain. Nature 508(7495):207–214. https://doi.org/10.1038/nature13186
Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I, Nitschko H, Busch U et al (2012) Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods 23(1):18–28. https://doi.org/10.1089/hgtb.2011.034
Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Kurosawa T (2013) Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp Anim 62(3):173–180. https://doi.org/10.1538/expanim.62.173
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates. Academic Press
Rodgers RJ, Ishii Y, Halford JC, Blundell JE (2002) Orexins and appetite regulation. Neuropeptides 36(5):303–325. https://doi.org/10.1016/s0143-4179(02)00085-9
Hohmann JG, Krasnow SM, Teklemichael DN, Clifton DK, Wynick D, Steiner RA (2003) Neuroendocrine profiles in galanin-overexpressing and knockout mice. Neuroendocrinology 77(6):354–366. https://doi.org/10.1159/000071308
Adams AC, Clapham JC, Wynick D, Speakman JR (2008) Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J Neuroendocrinol 20(2):199–206. https://doi.org/10.1111/j.1365-2826.2007.01638.x
Tachibana T, Mori M, Khan MS, Ueda H, Sugahara K, Hiramatsu K (2008) Central administration of galanin stimulates feeding behavior in chicks. Comp Biochem Physiol A Mol Integr Physiol 151(4):637–640. https://doi.org/10.1016/j.cbpa.2008.08.001
Chen KS, Xu M, Zhang Z, Chang WC, Gaj T, Schaffer DV, Dan Y (2018) A hypothalamic switch for REM and non-REM sleep. Neuron 97 (5):1168–1176 e1164. https://doi.org/10.1016/j.neuron.2018.02.005
Patterson M, Murphy KG, Thompson EL, Smith KL, Meeran K, Ghatei MA, Bloom SR (2006) Microinjection of galanin-like peptide into the medial preoptic area stimulates food intake in adult male rats. J Neuroendocrinol 18(10):742–747. https://doi.org/10.1111/j.1365-2826.2006.01473.x
Lawrence CB, Williams T, Luckman SM (2003) Intracerebroventricular galanin-like peptide induces different brain activation compared with galanin. Endocrinology 144(9):3977–3984. https://doi.org/10.1210/en.2003-0391
Engel JA, Hjorth S, Svensson K, Carlsson A, Liljequist S (1984) Anticonflict effect of the putative serotonin receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). Eur J Pharmacol 105(3–4):365–368. https://doi.org/10.1016/0014-2999(84)90634-4
Bing O, Moller C, Engel JA, Soderpalm B, Heilig M (1993) Anxiolytic-like action of centrally administered galanin. Neurosci Lett 164(1–2):17–20. https://doi.org/10.1016/0304-3940(93)90846-d
Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC et al (2003) Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 28(6):1031–1044. https://doi.org/10.1038/sj.npp.1300164
Karlsson RM, Holmes A (2006) Galanin as a modulator of anxiety and depression and a therapeutic target for affective disease. Amino Acids 31(3):231–239. https://doi.org/10.1007/s00726-006-0336-8
Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE (2013) PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology 38(5):702–715. https://doi.org/10.1016/j.psyneuen.2012.09.006
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK et al (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61(3):283–357. https://doi.org/10.1124/pr.109.001370
Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI (2004) Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol 476(4):388–413. https://doi.org/10.1002/cne.20231
Acknowledgements
We would like to thank Ms. Izumi Fujishima for their technical contribution and all the Joint Research Laboratory and the Division of Laboratory Animal Sciences, Kagoshima University, to help with animal care and the use of the facilities. The data in the present manuscript have been presented at the 95th annual meeting of the Japanese Society for Pharmacology (https://www.jstage.jst.go.jp/article/jpssuppl/95/0/95_1-P-004/_article/-char/ja/.). Still, no figures contained in the present paper have been published in a journal before.
Funding
We would like to express our deepest gratitude for a grant from the Kodama Memorial Fund for Medical Research for their research support and a grant from the Takeda Science Foundation. This work was supported by JSPS KAKENHI grant numbers JP20K07067, JP20H03429, JP18K07394, JP21K19335, JP20H00492, and JP19K07121; MEXT KAKENHI grant number JP18H05416; AMED grant number JP21dm0207117.
Author information
Authors and Affiliations
Contributions
YK carried out the experiments, performed behavioral studies, performed the statistical analysis, and wrote the manuscript. TTN, YS, and KH carried out the experiments. TY, TK, and AM conceived of and participated in the study’s design and wrote the manuscript. NS and HH participated in the study’s design and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experiments in the present study were approved by the Experimental Animal Research Committee of Kagoshima University (Approval numbers: MD16076, MD16116, and MD17054) and the Gene Recombination Experiment Safety Committee of Kagoshima University (Approval numbers: S28006, S28015, and S29007). All animal experiments were performed following the ARRIVE guidelines and the National Institutes of Health guide for the care and use of laboratory animals. The work described in the present study was carried out following The Code of Ethics of the World Medical Association for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm) and Uniform Requirements for manuscripts submitted to Biomedical Journals (http://www.icmje.org).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kambe, Y., Nguyen, T.T., Yasaka, T. et al. The Pivotal Role of Neuropeptide Crosstalk from Ventromedial-PACAP to Dorsomedial-Galanin in the Appetite Regulation in the Mouse Hypothalamus. Mol Neurobiol 60, 171–182 (2023). https://doi.org/10.1007/s12035-022-03084-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03084-y