Abstract
The endocannabinoid system (ECS) is composed of the endocannabinoid ligands anandamide (AEA) and 2-arachidonoylgycerol (2-AG), their target cannabinoid receptors (CB1 and CB2) and the enzymes involved in their synthesis and metabolism (N-acyltransferase and fatty acid amide hydrolase (FAAH) in the case of AEA and diacylglycerol lipase (DAGL) and monoacylglycerol lipase (MAGL) in the case of 2-AG). The origins of ECS dysfunction in major neuropsychiatric disorders remain to be determined, and this paper explores the possibility that they may be associated with chronically increased nitro-oxidative stress and activated immune-inflammatory pathways, and it examines the mechanisms which might be involved. Inflammation and nitro-oxidative stress are associated with both increased CB1 expression, via increased activity of the NADPH oxidases NOX4 and NOX1, and increased CNR1 expression and DNA methylation; and CB2 upregulation via increased pro-inflammatory cytokine levels, binding of the transcription factor Nrf2 to an antioxidant response element in the CNR2 promoter region and the action of miR-139. CB1 and CB2 have antagonistic effects on redox signalling, which may result from a miRNA-enabled negative feedback loop. The effects of inflammation and oxidative stress are detailed in respect of AEA and 2-AG levels, via effects on calcium homeostasis and phospholipase A2 activity; on FAAH activity, via nitrosylation/nitration of functional cysteine and/or tyrosine residues; and on 2-AG activity via effects on MGLL expression and MAGL. Finally, based on these detailed molecular neurobiological mechanisms, it is suggested that cannabidiol and dimethyl fumarate may have therapeutic potential for major depressive disorder, bipolar disorder and schizophrenia.


Similar content being viewed by others
Data Availability
Not applicable.
References
Tao R, Li C, Jaffe AE et al (2020) Cannabinoid receptor CNR1 expression and DNA methylation in human prefrontal cortex, hippocampus and caudate in brain development and schizophrenia. Transl Psychiatry 10:158. https://doi.org/10.1038/s41398-020-0832-8
Zou S, Kumar U (2018) Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 19:833. https://doi.org/10.3390/ijms19030833
Veilleux A, Di Marzo V, Silvestri C (2019) The expanded endocannabinoid system/endocannabinoidome as a potential target for treating diabetes mellitus. Curr Diab Rep 19:117. https://doi.org/10.1007/s11892-019-1248-9
Lipina C, Hundal HS (2017) The endocannabinoid system: ‘NO’ longer anonymous in the control of nitrergic signalling? J Mol Cell Biol 9:91–103. https://doi.org/10.1093/jmcb/mjx008
Lipina C, Hundal HS (2016) Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol 6:150276. https://doi.org/10.1098/rsob.150276
Paloczi J, Varga ZV, Hasko G, Pacher P (2018) Neuroprotection in oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxid Redox Signal 29:75–108. https://doi.org/10.1089/ars.2017.7144
Lisboa SF Gomes FV Silva AL et al (2015) Increased contextual fear conditioning in iNOS knockout mice: additional evidence for the involvement of nitric oxide in stress-related disorders and contribution of the endocannabinoid system. Int J Neuropsychopharmacol 18:pyv005–pyv005. https://doi.org/10.1093/ijnp/pyv005
Kim SH, Won SJ, Mao XO et al (2006) Role for neuronal nitric-oxide synthase in cannabinoid-induced neurogenesis. J Pharmacol Exp Ther 319:150–154. https://doi.org/10.1124/jpet.106.107698
Carracedo A, Geelen MJH, Diez M et al (2004) Ceramide sensitizes astrocytes to oxidative stress: protective role of cannabinoids. Biochem J 380:435–440. https://doi.org/10.1042/bj20031714
Mnich K, Finn DP, Dowd E, Gorman AM (2010) Inhibition by anandamide of 6-hydroxydopamine-induced cell death in PC12 cells. Int J Cell Biol 2010:e818497. https://doi.org/10.1155/2010/818497
Aso E, Juvés S, Maldonado R, Ferrer I (2013) CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J Alzheimers Dis 35:847–858. https://doi.org/10.3233/JAD-130137
Ribeiro R, Wen J, Li S, Zhang Y (2013) Involvement of ERK1/2, cPLA2 and NF-κB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat 100–101:1–14. https://doi.org/10.1016/j.prostaglandins.2012.11.003
Kim SH, Won SJ, Mao XO et al (2006) Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity. Mol Pharmacol 69:691–696. https://doi.org/10.1124/mol.105.016428
Kruk-Slomka M, Dzik A, Budzynska B, Biala G (2017) Endocannabinoid system: the direct and indirect involvement in the memory and learning processes—a short review. Mol Neurobiol 54:8332–8347. https://doi.org/10.1007/s12035-016-0313-5
Wang M, Abais JM, Meng N et al (2014) Upregulation of cannabinoid receptor-1 and fibrotic activation of mouse hepatic stellate cells during Schistosoma J. infection: role of NADPH oxidase. Free Radic Biol Med 71:109–120. https://doi.org/10.1016/j.freeradbiomed.2014.03.015
Ambrożewicz E, Wójcik P, Wroński A et al (2018) Pathophysiological alterations of redox signaling and endocannabinoid system in granulocytes and plasma of psoriatic patients. Cells 7:159. https://doi.org/10.3390/cells7100159
Li X, Han D, Tian Z et al (2016) Activation of cannabinoid receptor type II by AM1241 ameliorates myocardial fibrosis via Nrf2-mediated inhibition of TGF-β1/Smad3 pathway in myocardial infarction mice. Cell Physiol Biochem 39:1521–1536. https://doi.org/10.1159/000447855
Matthews AT, Lee JH, Borazjani A et al (2016) Oxyradical stress increases the biosynthesis of 2-arachidonoylglycerol: involvement of NADPH oxidase. Am J Physiol-Cell Physiol 311:C960–C974. https://doi.org/10.1152/ajpcell.00251.2015
Li R, Huang Z, Luo J et al (2020) Downregulation of the CB1-mediated endocannabinoid signaling underlies D-galactose-induced memory impairment. Front Mol Neurosci 13:130. https://doi.org/10.3389/fnmol.2020.00130
Flatow J, Buckley P, Miller BJ (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74:400–409. https://doi.org/10.1016/j.biopsych.2013.03.018
Liu T, Zhong S, Liao X et al (2015) A meta-analysis of oxidative stress markers in depression. PLoS ONE 10:e0138904. https://doi.org/10.1371/journal.pone.0138904
Morris G, Puri BK, Walker AJ et al (2019) Shared pathways for neuroprogression and somatoprogression in neuropsychiatric disorders. Neurosci Biobehav Rev 107:862–882. https://doi.org/10.1016/j.neubiorev.2019.09.025
Morris G, Stubbs B, Köhler CA et al (2018) The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Med Rev 41:255–265. https://doi.org/10.1016/j.smrv.2018.03.007
Morris G, Walder K, McGee SL et al (2017) A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev 74:1–20. https://doi.org/10.1016/j.neubiorev.2017.01.014
Maes M (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry 35:664–675. https://doi.org/10.1016/j.pnpbp.2010.06.014
Fillman SG, Weickert TW, Lenroot RK et al (2016) Elevated peripheral cytokines characterize a subgroup of people with schizophrenia displaying poor verbal fluency and reduced Broca’s area volume. Mol Psychiatry 21:1090–1098. https://doi.org/10.1038/mp.2015.90
Boerrigter D, Weickert TW, Lenroot R et al (2017) Using blood cytokine measures to define high inflammatory biotype of schizophrenia and schizoaffective disorder. J Neuroinflammation 14:188. https://doi.org/10.1186/s12974-017-0962-y
Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. https://doi.org/10.1038/mp.2016.3
Modabbernia A, Taslimi S, Brietzke E, Ashrafi M (2013) Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry 74:15–25. https://doi.org/10.1016/j.biopsych.2013.01.007
Maes M, Berk M, Goehler L et al (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66. https://doi.org/10.1186/1741-7015-10-66
Berk M, Williams LJ, Jacka FN et al (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:1–16. https://doi.org/10.1186/1741-7015-11-200
Moylan S, Maes M, Wray NR, Berk M (2013) The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18:595–606. https://doi.org/10.1038/mp.2012.33
Herkenham M, Lynn AB, Little MD et al (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87:1932–1936. https://doi.org/10.1073/pnas.87.5.1932
Azad SC, Kurz J, Marsicano G et al (2008) Activation of CB1 specifically located on GABAergic interneurons inhibits LTD in the lateral amygdala. Learn Mem 15:143–152. https://doi.org/10.1101/lm.741908
Häring M, Marsicano G, Lutz B, Monory K (2007) Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 146:1212–1219. https://doi.org/10.1016/j.neuroscience.2007.02.021
Hermann H, Marsicano G, Lutz B (2002) Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109:451–460. https://doi.org/10.1016/S0306-4522(01)00509-7
Oropeza VC, Mackie K, Van Bockstaele EJ (2007) Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res 1127:36–44. https://doi.org/10.1016/j.brainres.2006.09.110
Wei Y, Wang X, Wang L (2009) Presence and regulation of cannabinoid receptors in human retinal pigment epithelial cells. Mol Vis 15:1243–1251
Batkai S, Osei-Hyiaman D, Pan H et al (2007) Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21:1788–1800. https://doi.org/10.1096/fj.06-7451com
Yang H, Zhang J, Andreasson K, Chen C (2008) COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci 37:682–695. https://doi.org/10.1016/j.mcn.2007.12.019
Lee CH, Yoo K-Y, Choi JH et al (2010) Cyclooxygenase-2 immunoreactivity and protein level in the gerbil hippocampus during normal aging. Neurochem Res 35:99–106. https://doi.org/10.1007/s11064-009-0034-5
Jean-Gilles L, Braitch M, Latif ML et al (2015) Effects of pro-inflammatory cytokines on cannabinoid CB 1 and CB 2 receptors in immune cells. Acta Physiol 214:63–74. https://doi.org/10.1111/apha.12474
Jean-Gilles L, Gran B, Constantinescu CS (2010) Interaction between cytokines, cannabinoids and the nervous system. Immunobiology 215:606–610. https://doi.org/10.1016/j.imbio.2009.12.006
Loría F, Petrosino S, Mestre L et al (2008) Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur J Neurosci 28:633–641. https://doi.org/10.1111/j.1460-9568.2008.06377.x
Amaya F, Shimosato G, Kawasaki Y et al (2006) Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain 124:175–183. https://doi.org/10.1016/j.pain.2006.04.001
Börner C, Höllt V, Kraus J (2007) Activation of human T cells induces upregulation of cannabinoid receptor type 1 transcription. NeuroImmunoModulation 14:281–286. https://doi.org/10.1159/000117809
Börner C, Bedini A, Höllt V, Kraus J (2008) Analysis of promoter regions regulating basal and interleukin-4-inducible expression of the human CB1 receptor gene in T lymphocytes. Mol Pharmacol 73:1013–1019. https://doi.org/10.1124/mol.107.042945
Börner C, Höllt V, Sebald W, Kraus J (2007) Transcriptional regulation of the cannabinoid receptor type 1 gene in T cells by cannabinoids. J Leukoc Biol 81:336–343. https://doi.org/10.1189/jlb.0306224
Laprairie R, Kelly M, Denovan-Wright E (2012) The dynamic nature of type 1 cannabinoid receptor (CB1) gene transcription. Br J Pharmacol 167:1583–1595. https://doi.org/10.1111/j.1476-5381.2012.02175.x
Rotter A, Bayerlein K, Hansbauer M et al (2013) CB1 and CB2 receptor expression and promoter methylation in patients with cannabis dependence. Eur Addict Res 19:13–20. https://doi.org/10.1159/000338642
Mancino S, Burokas A, Gutiérrez-Cuesta J et al (2015) Epigenetic and proteomic expression changes promoted by eating addictive-like behavior. Neuropsychopharmacology 40:2788–2800. https://doi.org/10.1038/npp.2015.129
Morris G, Maes M, Murdjeva M, Puri BK (2019) Do human endogenous retroviruses contribute to multiple sclerosis, and if so, how? Mol Neurobiol 56:2590–2605. https://doi.org/10.1007/s12035-018-1255-x
Zhang C, Shu L, Kong A-NT (2015) MicroRNAs: new players in cancer prevention targeting Nrf2, oxidative stress and inflammatory pathways. Curr Pharmacol Rep 1:21–30. https://doi.org/10.1007/s40495-014-0013-7
Hou Q, Huang Y, Zhang C et al (2018) MicroRNA-200a targets cannabinoid receptor 1 and serotonin transporter to increase visceral hyperalgesia in diarrhea-predominant irritable bowel syndrome rats. J Neurogastroenterol Motil 24:656–668. https://doi.org/10.5056/jnm18037
Magenta A, Cencioni C, Fasanaro P et al (2011) miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ 18:1628–1639. https://doi.org/10.1038/cdd.2011.42
Xia Z, Meng F, Liu Y et al (2018) Decreased MiR-128-3p alleviates the progression of rheumatoid arthritis by up-regulating the expression of TNFAIP3. Biosci Rep 38:BSR20180540. https://doi.org/10.1042/BSR20180540
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65. https://doi.org/10.1038/365061a0
Núñez E, Benito C, Pazos MR et al (2004) Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synap N Y N 53:208–213. https://doi.org/10.1002/syn.20050
Ashton JC, Friberg D, Darlington CL, Smith PF (2006) Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett 396:113–116. https://doi.org/10.1016/j.neulet.2005.11.038
Madireddy S, Madireddy S (2022) Therapeutic interventions to mitigate mitochondrial dysfunction and oxidative stress-induced damage in patients with bipolar disorder. Int J Mol Sci 23:1844. https://doi.org/10.3390/ijms23031844
Matisz CE, Gruber AJ (2022) Neuroinflammatory remodeling of the anterior cingulate cortex as a key driver of mood disorders in gastrointestinal disease and disorders. Neurosci Biobehav Rev 133:104497. https://doi.org/10.1016/j.neubiorev.2021.12.020
Nogo D, Wilkialis L, Lui LMW et al (2021) Examining the association between inflammation and motivational anhedonia in neuropsychiatric disorders: a systematic review. Ann Clin Psychiatry Off J Am Acad Clin Psychiatr 33:193–206. https://doi.org/10.12788/acp.0034
Bishop JR, Zhang L, Lizano P (2022) Inflammation subtypes and translating inflammation-related genetic findings in schizophrenia and related psychoses: a perspective on pathways for treatment stratification and novel therapies. Harv Rev Psychiatry 30:59–70. https://doi.org/10.1097/HRP.0000000000000321
Dunleavy C Elsworthy RJ Upthegrove R et al (2022) Inflammation in first-episode psychosis: the contribution of inflammatory biomarkers to the emergence of negative symptoms, a systematic review and meta-analysis. Acta Psychiatr Scand. https://doi.org/10.1111/acps.13416
Murphy CE, Walker AK, Weickert CS (2021) Neuroinflammation in schizophrenia: the role of nuclear factor kappa B. Transl Psychiatry 11:528. https://doi.org/10.1038/s41398-021-01607-0
Maresz K, Carrier EJ, Ponomarev ED et al (2005) Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95:437–445. https://doi.org/10.1111/j.1471-4159.2005.03380.x
Racz I, Nadal X, Alferink J et al (2008) Interferon-gamma is a critical modulator of cb2 cannabinoid receptor signaling during neuropathic pain. J Neurosci 28:12136–12145. https://doi.org/10.1523/JNEUROSCI.3402-08.2008
Lou Z-Y, Chen C, He Q et al (2011) Targeting CB2 receptor as a neuroinflammatory modulator in experimental autoimmune encephalomyelitis. Mol Immunol 49:453–461. https://doi.org/10.1016/j.molimm.2011.09.016
Martín-Saldaña S, Trinidad A, Ramil E et al (2016) Spontaneous cannabinoid receptor 2 (CB2) expression in the cochlea of adult albino rat and its up-regulation after cisplatin treatment. PLoS ONE 11:e0161954. https://doi.org/10.1371/journal.pone.0161954
Ghosh S Sheth S Sheehan K et al (2018) The endocannabinoid/cannabinoid receptor 2 system protects against cisplatin-induced hearing loss. Front Cell Neurosci 12:
Wardyn JD, Ponsford AH, Sanderson CM (2015) Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans 43:621–626. https://doi.org/10.1042/BST20150014
Galán-Ganga M, del Río R, Jiménez-Moreno N et al (2020) Cannabinoid CB2 receptor modulation by the transcription factor NRF2 is specific in microglial cells. Cell Mol Neurobiol 40:167–177. https://doi.org/10.1007/s10571-019-00719-y
Agudelo M, Newton C, Widen R et al (2008) Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol 3:35–42. https://doi.org/10.1007/s11481-007-9088-9
Schroder AJ, Pavlidis P, Arimura A et al (2002) Cutting edge: STAT6 serves as a positive and negative regulator of gene expression in IL-4-stimulated B lymphocytes. J Immunol 168:996–1000. https://doi.org/10.4049/jimmunol.168.3.996
Sherwood TA, Nong L, Agudelo M et al (2009) Identification of transcription start sites and preferential expression of select CB2 transcripts in mouse and human B lymphocytes. J Neuroimmune Pharmacol 4:476. https://doi.org/10.1007/s11481-009-9169-z
Kim HJ, Lim J, Jang Y-S et al (2017) Exogenous hydrogen peroxide induces lipid raft-mediated STAT-6 activation in T cells. Cell Physiol Biochem 42:2467–2480. https://doi.org/10.1159/000480210
Hirakawa S, Saito R, Ohara H et al (2011) Dual oxidase 1 induced by Th2 cytokines promotes STAT6 phosphorylation via oxidative inactivation of protein tyrosine phosphatase 1B in human epidermal keratinocytes. J Immunol 186:4762–4770. https://doi.org/10.4049/jimmunol.1000791
Möhnle P, Schütz SV, Schmidt M et al (2014) MicroRNA-665 is involved in the regulation of the expression of the cardioprotective cannabinoid receptor CB2 in patients with severe heart failure. Biochem Biophys Res Commun 451:516–521. https://doi.org/10.1016/j.bbrc.2014.08.008
Tang Y Bao JS Su JH Huang W (2017) MicroRNA-139 modulates Alzheimer’s-associated pathogenesis in SAMP8 mice by targeting cannabinoid receptor type 2. Genet Mol Res 16:. https://doi.org/10.4238/gmr16019166
Li T, Liang S, Zhang Y, Chen Y (2015) Effects of microRNA-139 on myocardial cell injury induced by oxidative stress. Int J Clin Exp Med 8:19994–20001
Shi Y-Y, Cui H-F, Qin B-J (2019) Monomethyl fumarate protects cerebral hemorrhage injury in rats via activating microRNA-139/Nrf2 axis. Eur Rev Med Pharmacol Sci 23:5012–5019. https://doi.org/10.26355/eurrev_201906_18093
Juknat A, Gao F, Coppola G et al (2019) miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia—effect of cannabinoids. PLoS ONE 14:e0212039. https://doi.org/10.1371/journal.pone.0212039
Blasco-Baque V, Coupé B, Fabre A et al (2017) Associations between hepatic miRNA expression, liver triacylglycerols and gut microbiota during metabolic adaptation to high-fat diet in mice. Diabetologia 60:690–700. https://doi.org/10.1007/s00125-017-4209-3
Chiarlone A, Börner C, Martín-Gómez L et al (2016) MicroRNA let-7d is a target of cannabinoid CB1 receptor and controls cannabinoid signaling. Neuropharmacology 108:345–352. https://doi.org/10.1016/j.neuropharm.2016.05.007
Dinu AR, Rogobete AF, Bratu T et al (2020) Cannabis sativa revisited—crosstalk between microRNA expression, inflammation, oxidative stress, and endocannabinoid response system in critically ill patients with sepsis. Cells 9:307. https://doi.org/10.3390/cells9020307
He J, Jiang B-H (2016) Interplay between reactive oxygen species and microRNAs in cancer. Curr Pharmacol Rep 2:82–90. https://doi.org/10.1007/s40495-016-0051-4
Morris G, Puri BK, Olive L et al (2020) Endothelial dysfunction in neuroprogressive disorders—causes and suggested treatments. BMC Med 18:1–31. https://doi.org/10.1186/s12916-020-01749-w
Garavelli S De Rosa V de Candia P (2018) The multifaceted interface between cytokines and microRNAs: an ancient mechanism to regulate the good and the bad of inflammation. Front Immunol 9:
Lin Y-H (2019) MicroRNA networks modulate oxidative stress in cancer. Int J Mol Sci 20:4497. https://doi.org/10.3390/ijms20184497
Gu S, Lai Y, Chen H et al (2017) miR-155 mediates arsenic trioxide resistance by activating Nrf2 and suppressing apoptosis in lung cancer cells. Sci Rep 7:12155. https://doi.org/10.1038/s41598-017-06061-x
Stella N (2009) Endocannabinoid signaling in microglial cells. Neuropharmacology 56:244–253. https://doi.org/10.1016/j.neuropharm.2008.07.037
Witting A, Walter L, Wacker J et al (2004) P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc Natl Acad Sci 101:3214–3219. https://doi.org/10.1073/pnas.0306707101
Sido JM, Nagarkatti PS, Nagarkatti M (2016) Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur J Immunol 46:1472–1479. https://doi.org/10.1002/eji.201546181
Liu J, Bátkai S, Pacher P et al (2003) Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-κB independently of platelet-activating factor. J Biol Chem 278:45034–45039. https://doi.org/10.1074/jbc.M306062200
Maccarrone M, De Petrocellis L, Bari M et al (2001) Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch Biochem Biophys 393:321–328. https://doi.org/10.1006/abbi.2001.2500
Dotsey EY, Jung K-M, Basit A et al (2015) Peroxide-dependent MGL sulfenylation regulates 2-AG-mediated endocannabinoid signaling in brain neurons. Chem Biol 22:619–628. https://doi.org/10.1016/j.chembiol.2015.04.013
Maccarrone M (2017) Metabolism of the endocannabinoid anandamide: open questions after 25 years. Front Mol Neurosci 10:
Placzek EA, Okamoto Y, Ueda N, Barker EL (2008) Mechanisms for recycling and biosynthesis of endogenous cannabinoids anandamide and 2-arachidonylglycerol. J Neurochem 107:987–1000. https://doi.org/10.1111/j.1471-4159.2008.05659.x
Walter L (2004) ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J Neurosci 24:8068–8074. https://doi.org/10.1523/JNEUROSCI.2419-04.2004
Görlach A, Bertram K, Hudecova S, Krizanova O (2015) Calcium and ROS: a mutual interplay. Redox Biol 6:260–271. https://doi.org/10.1016/j.redox.2015.08.010
Magenta A, Dellambra E, Ciarapica R, Capogrossi MC (2016) Oxidative stress, microRNAs and cytosolic calcium homeostasis. Cell Calcium 60:207–217. https://doi.org/10.1016/j.ceca.2016.04.002
Hegyi Z, Oláh T, Kőszeghy Á et al (2018) CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Sci Rep 8:10562. https://doi.org/10.1038/s41598-018-28763-6
Reisenberg M, Singh PK, Williams G, Doherty P (2012) The diacylglycerol lipases: structure, regulation and roles in and beyond endocannabinoid signalling. Philos Trans R Soc B Biol Sci 367:3264–3275. https://doi.org/10.1098/rstb.2011.0387
Baggelaar MP, Maccarrone M, van der Stelt M (2018) 2-arachidonoylglycerol: a signaling lipid with manifold actions in the brain. Prog Lipid Res 71:1–17. https://doi.org/10.1016/j.plipres.2018.05.002
Shonesy BC, Wang X, Rose KL et al (2013) CaMKII regulates diacylglycerol lipase-α and striatal endocannabinoid signaling. Nat Neurosci 16:456–463. https://doi.org/10.1038/nn.3353
Ludányi A, Hu SS-J, Yamazaki M et al (2011) Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience 174:50–63. https://doi.org/10.1016/j.neuroscience.2010.10.062
Jung K-M, Astarita G, Zhu C et al (2007) A key role for diacylglycerol lipase-α in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol 72:612–621. https://doi.org/10.1124/mol.107.037796
Walter L, Franklin A, Witting A et al (2002) Astrocytes in culture produce anandamide and other acylethanolamides. J Biol Chem 277:20869–20876. https://doi.org/10.1074/jbc.M110813200
Stella N, Piomelli D (2001) Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol 425:189–196. https://doi.org/10.1016/S0014-2999(01)01182-7
Ueda N, Tsuboi K, Uyama T (2013) Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J 280:1874–1894. https://doi.org/10.1111/febs.12152
Hussain Z, Uyama T, Kawai K et al (2018) Phosphatidylserine-stimulated production of N-acyl-phosphatidylethanolamines by Ca2+-dependent N-acyltransferase. Biochim Biophys Acta BBA - Mol Cell Biol Lipids 1863:493–502. https://doi.org/10.1016/j.bbalip.2018.02.002
Ogura Y, Parsons WH, Kamat SS, Cravatt BF (2016) A calcium-dependent acyltransferase that produces N-acyl phosphatidylethanolamines. Nat Chem Biol 12:669–671. https://doi.org/10.1038/nchembio.2127
Okamoto Y, Morishita J, Tsuboi K et al (2004) Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279:5298–5305. https://doi.org/10.1074/jbc.M306642200
Rahman IAS, Tsuboi K, Uyama T, Ueda N (2014) New players in the fatty acyl ethanolamide metabolism. Pharmacol Res 86:1–10. https://doi.org/10.1016/j.phrs.2014.04.001
Ibsen MS, Connor M, Glass M (2017) Cannabinoid CB 1 and CB 2 receptor signaling and bias. Cannabis Cannabinoid Res 2:48–60. https://doi.org/10.1089/can.2016.0037
Del Prete D, Checler F, Chami M (2014) Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9:21. https://doi.org/10.1186/1750-1326-9-21
Kushnir A, Wajsberg B, Marks AR (2018) Ryanodine receptor dysfunction in human disorders. Biochim Biophys Acta BBA - Mol Cell Res 1865:1687–1697. https://doi.org/10.1016/j.bbamcr.2018.07.011
Karagas NE, Venkatachalam K (2019) Roles for the endoplasmic reticulum in regulation of neuronal calcium homeostasis. Cells 8:1232. https://doi.org/10.3390/cells8101232
Zhang I, Hu H (2020) Store-operated calcium channels in physiological and pathological states of the nervous system. Front Cell Neurosci 14:600758. https://doi.org/10.3389/fncel.2020.600758
Mei Y, Barrett JE, Hu H (2018) Calcium release-activated calcium channels and pain. Cell Calcium 74:180–185. https://doi.org/10.1016/j.ceca.2018.07.009
Wang R, Tu S, Zhang J, Shao A (2020) Roles of TRP channels in neurological diseases. Oxid Med Cell Longev 2020:1–13. https://doi.org/10.1155/2020/7289194
Nascimento Da Conceicao V Sun Y Zboril EK et al (2019) Loss of Ca2+ entry via Orai-TRPC1 induces ER stress that initiates immune activation in macrophage cells. J Cell Sci jcs.237610. https://doi.org/10.1242/jcs.237610
Vigont V Kolobkova Y Skopin A et al (2015) Both Orai1 and TRPC1 are involved in excessive store-operated calcium entry in striatal neurons expressing mutant huntingtin exon 1. Front Physiol 6:. https://doi.org/10.3389/fphys.2015.00337
Asghar MY, Törnquist K (2020) Transient receptor potential canonical (TRPC) channels as modulators of migration and invasion. Int J Mol Sci 21:1739. https://doi.org/10.3390/ijms21051739
Britzolaki A, Saurine J, Klocke B, Pitychoutis PM (2020) A role for SERCA pumps in the neurobiology of neuropsychiatric and neurodegenerative disorders. In: Islam MdS (ed) Calcium Signaling. Springer International Publishing, Cham, pp 131–161
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189:803–811. https://doi.org/10.1016/j.juro.2012.05.078
Lock JT, Sinkins WG, Schilling WP (2012) Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J Physiol 590:3431–3447. https://doi.org/10.1113/jphysiol.2012.232645
Bánsághi S, Golenár T, Madesh M et al (2014) Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J Biol Chem 289:8170–8181. https://doi.org/10.1074/jbc.M113.504159
Bhardwaj R, Hediger MA, Demaurex N (2016) Redox modulation of STIM-ORAI signaling. Cell Calcium 60:142–152. https://doi.org/10.1016/j.ceca.2016.03.006
Grupe M, Myers G, Penner R, Fleig A (2010) Activation of store-operated ICRAC by hydrogen peroxide. Cell Calcium 48:1–9. https://doi.org/10.1016/j.ceca.2010.05.005
Gibhardt CS, Cappello S, Bhardwaj R et al (2020) Oxidative stress-induced STIM2 cysteine modifications suppress store-operated calcium entry. Cell Rep 33:108292. https://doi.org/10.1016/j.celrep.2020.108292
Bogeski I Kummerow C Al-Ansary D et al (2010) Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci Signal 3:. https://doi.org/10.1126/scisignal.2000672
Gandhirajan RK Meng S Chandramoorthy HC et al (2013) Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest JCI65647. https://doi.org/10.1172/JCI65647
Alansary D, Bogeski I, Niemeyer BA (2015) Facilitation of Orai3 targeting and store-operated function by Orai1. Biochim Biophys Acta BBA - Mol Cell Res 1853:1541–1550. https://doi.org/10.1016/j.bbamcr.2015.03.007
Song MY, Makino A, Yuan JX-J (2011) Role of reactive oxygen species and redox in regulating the function of transient receptor potential channels. Antioxid Redox Signal 15:1549–1565. https://doi.org/10.1089/ars.2010.3648
Weissmann N, Sydykov A, Kalwa H et al (2012) Activation of TRPC6 channels is essential for lung ischaemia–reperfusion induced oedema in mice. Nat Commun 3:649. https://doi.org/10.1038/ncomms1660
Jiang Q, Fu X, Tian L et al (2014) NOX4 mediates BMP4-induced upregulation of TRPC1 and 6 protein expressions in distal pulmonary arterial smooth muscle cells. PLoS ONE 9:e107135. https://doi.org/10.1371/journal.pone.0107135
Poteser M, Graziani A, Rosker C et al (2006) TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. J Biol Chem 281:13588–13595. https://doi.org/10.1074/jbc.M512205200
Vazquez G (2012) TRPC channels as prospective targets in atherosclerosis: terra incognita. Front Biosci Sch Ed 4:157–166. https://doi.org/10.2741/258
Sharov VS, Dremina ES, Galeva NA et al (2006) Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC–electrospray-tandem MS: selective protein oxidation during biological aging. Biochem J 394:605–615. https://doi.org/10.1042/BJ20051214
Lancel S, Qin F, Lennon SL et al (2010) Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Gαq-overexpressing mice. Circ Res 107:228–232. https://doi.org/10.1161/CIRCRESAHA.110.217570
Qin F Siwik DA Lancel S et al (2013) Hydrogen peroxide–mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc 2:. https://doi.org/10.1161/JAHA.113.000184
Tong X, Ying J, Pimentel DR et al (2008) High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol 44:361–369. https://doi.org/10.1016/j.yjmcc.2007.10.022
Zaidi A (2010) Plasma membrane Ca 2+ -ATPases: targets of oxidative stress in brain aging and neurodegeneration. World J Biol Chem 1:271. https://doi.org/10.4331/wjbc.v1.i9.271
Kim MJ, Choi KJ, Yoon MN et al (2018) Hydrogen peroxide inhibits Ca 2+ efflux through plasma membrane Ca 2+ -ATPase in mouse parotid acinar cells. Korean J Physiol Pharmacol 22:215. https://doi.org/10.4196/kjpp.2018.22.2.215
Berridge MJ (2014) Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res 357:477–492. https://doi.org/10.1007/s00441-014-1806-z
Park SJ, Jeong J, Park Y-U et al (2015) Disrupted-in-schizophrenia-1 (DISC1) regulates endoplasmic reticulum calcium dynamics. Sci Rep 5:8694. https://doi.org/10.1038/srep08694
Vidal-Domènech F Riquelme G Pinacho R et al (2020) Calcium-binding proteins are altered in the cerebellum in schizophrenia. Neuroscience
Harrison PJ, Hall N, Mould A et al (2021) Cellular calcium in bipolar disorder: systematic review and meta-analysis. Mol Psychiatry 26:4106–4116. https://doi.org/10.1038/s41380-019-0622-y
Steardo L, Luciano M, Sampogna G et al (2020) Clinical severity and calcium metabolism in patients with bipolar disorder. Brain Sci 10:417. https://doi.org/10.3390/brainsci10070417
Deutschenbaur L, Beck J, Kiyhankhadiv A et al (2016) Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog Neuropsychopharmacol Biol Psychiatry 64:325–333. https://doi.org/10.1016/j.pnpbp.2015.02.015
Grützner TM, Listunova L, Fabian GA et al (2018) Serum calcium levels and neuropsychological performance in depression and matched healthy controls: reversal of correlation a marker of the aging cognitive clock? Psychoneuroendocrinology 91:198–205. https://doi.org/10.1016/j.psyneuen.2018.03.012
Al-Dujaili AH, Al-Hakeim HK, Twayej AJ, Maes M (2019) Total and ionized calcium and magnesium are significantly lowered in drug-naïve depressed patients: effects of antidepressants and associations with immune activation. Metab Brain Dis 34:1493–1503. https://doi.org/10.1007/s11011-019-00458-5
Secondo A, Bagetta G, Amantea D (2018) On the role of store-operated calcium entry in acute and chronic neurodegenerative diseases. Front Mol Neurosci 11:87. https://doi.org/10.3389/fnmol.2018.00087
Sirabella R, Secondo A, Pannaccione A et al (2009) Anoxia-induced NF-kappaB-dependent upregulation of NCX1 contributes to Ca2+ refilling into endoplasmic reticulum in cortical neurons. Stroke 40:922–929. https://doi.org/10.1161/STROKEAHA.108.531962
Zhang M, Song J-N, Wu Y et al (2014) Suppression of STIM1 in the early stage after global ischemia attenuates the injury of delayed neuronal death by inhibiting store-operated calcium entry-induced apoptosis in rats. NeuroReport 25:507–513. https://doi.org/10.1097/WNR.0000000000000127
Sun S, Zhang H, Liu J et al (2014) Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron 82:79–93. https://doi.org/10.1016/j.neuron.2014.02.019
Zhang H, Wu L, Pchitskaya E et al (2015) Neuronal store-operated calcium entry and mushroom spine loss in amyloid precursor protein knock-in mouse model of Alzheimer’s Disease. J Neurosci Off J Soc Neurosci 35:13275–13286. https://doi.org/10.1523/JNEUROSCI.1034-15.2015
Selvaraj S, Sun Y, Watt JA et al (2012) Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest 122:1354–1367. https://doi.org/10.1172/JCI61332
Li B, Xiao L, Wang ZY, Zheng PS (2014) Knockdown of STIM1 inhibits 6-hydroxydopamine-induced oxidative stress through attenuating calcium-dependent ER stress and mitochondrial dysfunction in undifferentiated PC12 cells. Free Radic Res 48:758–768. https://doi.org/10.3109/10715762.2014.905687
Li X, Chen W, Zhang L et al (2013) Inhibition of store-operated calcium entry attenuates MPP(+)-induced oxidative stress via preservation of mitochondrial function in PC12 cells: involvement of Homer1a. PLoS ONE 8:e83638. https://doi.org/10.1371/journal.pone.0083638
Zhou Q, Yen A, Rymarczyk G et al (2016) Impairment of PARK14-dependent Ca(2+) signalling is a novel determinant of Parkinson’s disease. Nat Commun 7:10332. https://doi.org/10.1038/ncomms10332
Pathak T, Agrawal T, Richhariya S et al (2015) Store-operated calcium entry through Orai is required for transcriptional maturation of the flight circuit in drosophila. J Neurosci Off J Soc Neurosci 35:13784–13799. https://doi.org/10.1523/JNEUROSCI.1680-15.2015
Wu J, Shih H-P, Vigont V et al (2011) Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington’s disease treatment. Chem Biol 18:777–793. https://doi.org/10.1016/j.chembiol.2011.04.012
Wu J, Ryskamp DA, Liang X et al (2016) Enhanced store-operated calcium entry leads to striatal synaptic loss in a Huntington’s disease mouse model. J Neurosci Off J Soc Neurosci 36:125–141. https://doi.org/10.1523/JNEUROSCI.1038-15.2016
Tang T-S, Slow E, Lupu V et al (2005) Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci U S A 102:2602–2607. https://doi.org/10.1073/pnas.0409402102
Tang T-S, Guo C, Wang H et al (2009) Neuroprotective effects of inositol 1,4,5-trisphosphate receptor C-terminal fragment in a Huntington’s disease mouse model. J Neurosci Off J Soc Neurosci 29:1257–1266. https://doi.org/10.1523/JNEUROSCI.4411-08.2009
Vigont VA, Zimina OA, Glushankova LN et al (2014) STIM1 protein activates store-operated calcium channels in cellular model of Huntington’s disease. Acta Naturae 6:40–47
Czeredys M, Maciag F, Methner A, Kuznicki J (2017) Tetrahydrocarbazoles decrease elevated SOCE in medium spiny neurons from transgenic YAC128 mice, a model of Huntington’s disease. Biochem Biophys Res Commun 483:1194–1205. https://doi.org/10.1016/j.bbrc.2016.08.106
Loffredo S Borriello F Iannone R et al (2017) Group V secreted phospholipase A2 induces the release of proangiogenic and antiangiogenic factors by human neutrophils. Front Immunol 8:. https://doi.org/10.3389/fimmu.2017.00443
Murakami M (2017) Lipoquality control by phospholipase A2 enzymes. Proc Jpn Acad Ser B 93:677–702. https://doi.org/10.2183/pjab.93.043
Quach ND, Arnold RD, Cummings BS (2014) Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochem Pharmacol 90:338–348. https://doi.org/10.1016/j.bcp.2014.05.022
Sun GY, Chuang DY, Zong Y et al (2014) Role of cytosolic phospholipase A2 in oxidative and inflammatory signaling pathways in different cell types in the central nervous system. Mol Neurobiol 50:6–14. https://doi.org/10.1007/s12035-014-8662-4
Sun GY, He Y, Chuang DY et al (2012) Integrating cytosolic phospholipase A2 with oxidative/nitrosative signaling pathways in neurons: a novel therapeutic strategy for AD. Mol Neurobiol 46:85–95. https://doi.org/10.1007/s12035-012-8261-1
Shelat PB, Chalimoniuk M, Wang J-H et al (2008) Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A 2 in cortical neurons. J Neurochem 106:45–55. https://doi.org/10.1111/j.1471-4159.2008.05347.x
Simon V, Cota D (2017) Mechanisms in endocrinology: endocannabinoids and metabolism: past, present and future. Eur J Endocrinol 176:R309–R324. https://doi.org/10.1530/EJE-16-1044
Leslie CC (2015) Cytosolic phospholipase A2: physiological function and role in disease. J Lipid Res 56:1386–1402. https://doi.org/10.1194/jlr.R057588
Sanchez-Mejia RO, Mucke L (2010) Phospholipase A2 and arachidonic acid in Alzheimer’s disease. Biochim Biophys Acta BBA - Mol Cell Biol Lipids 1801:784–790. https://doi.org/10.1016/j.bbalip.2010.05.013
Ng CY, Kannan S, Chen YJ et al (2017) A new generation of arachidonic acid analogues as potential neurological agent targeting cytosolic phospholipase A2. Sci Rep 7:13683. https://doi.org/10.1038/s41598-017-13996-8
Ding N Jiang J Tian H et al (2020) Benign regulation of the astrocytic phospholipase A2-arachidonic acid pathway: the underlying mechanism of the beneficial effects of manual acupuncture on CBF. Front Neurosci 13:
Murakami M, Sato H, Taketomi Y (2020) Updating phospholipase A2 biology. Biomolecules 10:1457. https://doi.org/10.3390/biom10101457
Bisogno T, Melck D, Petrocellis L, Marzo V (2008) Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem 72:2113–2119. https://doi.org/10.1046/j.1471-4159.1999.0722113.x
Basavarajappa B (2007) Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett 14:237–246. https://doi.org/10.2174/092986607780090829
Sun Y-X, Tsuboi K, Okamoto Y et al (2004) Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J 380:749–756. https://doi.org/10.1042/bj20040031
Chang JP-C, Guu T-W, Chen Y-C et al (2018) BanI polymorphism of cytosolic phospholipase A2 gene and somatic symptoms in medication-free acute depressed patients. Prostaglandins Leukot Essent Fatty Acids 136:111–115. https://doi.org/10.1016/j.plefa.2017.01.001
Gałecki P, Gałecka E, Maes M et al (2012) The expression of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients with recurrent depressive disorder. J Affect Disord 138:360–366. https://doi.org/10.1016/j.jad.2012.01.016
Pae C-U, Yu H-S, Kim J-J et al (2004) Bani polymorphism of the cytosolic phospholipase a2 gene and mood disorders in the Korean population. Neuropsychobiology 49:185–188. https://doi.org/10.1159/000077364
Kim H-W, Rapoport SI, Rao JS (2011) Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry 16:419–428. https://doi.org/10.1038/mp.2009.137
Ross BM, Hughes B, Kish SJ, Warsh JJ (2006) Serum calcium-independent phospholipase A2 activity in bipolar affective disorder. Bipolar Disord 8:265–270. https://doi.org/10.1111/j.1399-5618.2006.00299.x
Macdonald DJ, Boyle RM, Glen ACA et al (2004) The investigation of cytosolic phospholipase A2 using ELISA. Prostaglandins Leukot Essent Fatty Acids 70:377–381. https://doi.org/10.1016/j.plefa.2003.12.013
Law MH, Cotton RGH, Berger GE (2006) The role of phospholipases A2 in schizophrenia. Mol Psychiatry 11:547–556. https://doi.org/10.1038/sj.mp.4001819
Hamaguchi M, Wu HN, Tanaka M et al (2019) A case series of the dynamics of lipid mediators in patients with sepsis. Acute Med Surg 6:413–418. https://doi.org/10.1002/ams2.443
Tanaka M, Yanagihara I, Takahashi H et al (2007) The mRNA expression of fatty acid amide hydrolase in human whole blood correlates with sepsis. J Endotoxin Res 13:35–38. https://doi.org/10.1177/0968051907078607
Aisemberg J, Vercelli C, Wolfson M et al (2010) Inflammatory agents involved in septic miscarriage. NeuroImmunoModulation 17:150–152. https://doi.org/10.1159/000258710
Wolfson ML, Aisemberg J, Correa F, Franchi AM (2017) Peripheral blood mononuclear cells infiltration downregulates decidual FAAH activity in an LPS-induced embryo resorption model: NO modulates decidual FAAH activity. J Cell Physiol 232:1441–1447. https://doi.org/10.1002/jcp.25640
Maccarrone M, Bari M, Di Rienzo M et al (2003) Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. J Biol Chem 278:32726–32732. https://doi.org/10.1074/jbc.M302123200
Butturini E, Carcereri de Prati A, Mariotto S (2020) Redox regulation of STAT1 and STAT3 signaling. Int J Mol Sci 21:7034. https://doi.org/10.3390/ijms21197034
Rakhshandehroo M, Sanderson LM, Matilainen M et al (2007) Comprehensive analysis of PPAR α - dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res 2007:1–13. https://doi.org/10.1155/2007/26839
Chon S-H, Zhou YX, Dixon JL, Storch J (2007) Intestinal monoacylglycerol metabolism. J Biol Chem 282:33346–33357. https://doi.org/10.1074/jbc.M706994200
Blanquicett C, Kang B-Y, Ritzenthaler JD et al (2010) Oxidative stress modulates PPAR gamma in vascular endothelial cells. Free Radic Biol Med 48:1618–1625. https://doi.org/10.1016/j.freeradbiomed.2010.03.007
Almeida M, Ambrogini E, Han L et al (2009) Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem 284:27438–27448. https://doi.org/10.1074/jbc.M109.023572
Barger PM, Browning AC, Garner AN, Kelly DP (2001) p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem 276:44495–44501. https://doi.org/10.1074/jbc.M105945200
Ibarra-Lara L, Hong E, Soria-Castro E et al (2012) Clofibrate PPARα activation reduces oxidative stress and improves ultrastructure and ventricular hemodynamics in no-flow myocardial ischemia. J Cardiovasc Pharmacol 60:323–334. https://doi.org/10.1097/FJC.0b013e31826216ed
Billiet L, Furman C, Cuaz-Pérolin C et al (2008) Thioredoxin-1 and its natural inhibitor, vitamin D3 up-regulated protein 1, are differentially regulated by PPARalpha in human macrophages. J Mol Biol 384:564–576. https://doi.org/10.1016/j.jmb.2008.09.061
Kim T, Yang Q (2013) Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World J Cardiol 5:164–174. https://doi.org/10.4330/wjc.v5.i6.164
Morris G, Walder KR, Berk M et al (2020) The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: can we explain it and can we treat it? Mol Biol Rep 47:5587–5620. https://doi.org/10.1007/s11033-020-05590-5
King A, Lodola A, Carmi C et al (2009) A critical cysteine residue in monoacylglycerol lipase is targeted by a new class of isothiazolinone-based enzyme inhibitors. Br J Pharmacol 157:974–983. https://doi.org/10.1111/j.1476-5381.2009.00276.x
Deng H, Li W (2020) Monoacylglycerol lipase inhibitors: modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders. Acta Pharm Sin B 10:582–602. https://doi.org/10.1016/j.apsb.2019.10.006
Grabner GF, Zimmermann R, Schicho R, Taschler U (2017) Monoglyceride lipase as a drug target: at the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther 175:35–46. https://doi.org/10.1016/j.pharmthera.2017.02.033
Mulvihill MM, Nomura DK (2013) Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci 92:492–497. https://doi.org/10.1016/j.lfs.2012.10.025
Sumislawski JJ, Ramikie TS, Patel S (2011) Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology 36:2750–2761. https://doi.org/10.1038/npp.2011.166
Ogasawara D, Deng H, Viader A et al (2016) Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc Natl Acad Sci 113:26–33. https://doi.org/10.1073/pnas.1522364112
Chanda PK, Gao Y, Mark L et al (2010) Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol 78:996–1003. https://doi.org/10.1124/mol.110.068304
Schlosburg JE, Blankman JL, Long JZ et al (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13:1113–1119. https://doi.org/10.1038/nn.2616
Wamsteeker JI, Bains JS (2011) Monoacylglycerol lipase: stopping surplus at the synapse. J Physiol 589:5335–5336. https://doi.org/10.1113/jphysiol.2011.221135
Villares J (2007) Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience 145:323–334. https://doi.org/10.1016/j.neuroscience.2006.11.012
Hirvonen J, Goodwin RS, Li C-T et al (2012) Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17:642–649. https://doi.org/10.1038/mp.2011.82
Sim-Selley LJ (2003) Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol 15:91–119. https://doi.org/10.1615/CritRevNeurobiol.v15.i2.10
Burston JJ, Wiley JL, Craig AA et al (2010) Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Δ9-tetrahydrocannabinol exposure: THC and adolescent CB1 receptor desensitization. Br J Pharmacol 161:103–112. https://doi.org/10.1111/j.1476-5381.2010.00870.x
Lazenka MF, Selley DE, Sim-Selley LJ (2013) Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci 92:446–452. https://doi.org/10.1016/j.lfs.2012.08.023
Makara JK, Mor M, Fegley D et al (2005) Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci 8:1139–1141. https://doi.org/10.1038/nn1521
Hashimotodani Y, Ohno-Shosaku T, Kano M (2007) Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci 27:1211–1219. https://doi.org/10.1523/JNEUROSCI.4159-06.2007
Pan B, Wang W, Long JZ et al (2009) Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d ][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther 331:591–597. https://doi.org/10.1124/jpet.109.158162
Dubois CJ, Fawcett-Patel J, Katzman PA, Liu SJ (2020) Inhibitory neurotransmission drives endocannabinoid degradation to promote memory consolidation. Nat Commun 11:6407. https://doi.org/10.1038/s41467-020-20121-3
Nomura DK, Morrison BE, Blankman JL et al (2011) Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science 334:809–813. https://doi.org/10.1126/science.1209200
Monteleone P, Bifulco M, Maina G et al (2010) Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol Res 61:400–404. https://doi.org/10.1016/j.phrs.2010.01.002
Navarrete F, García-Gutiérrez MS, Jurado-Barba R et al (2020) Endocannabinoid system components as potential biomarkers in psychiatry. Front Psychiatry 11:315. https://doi.org/10.3389/fpsyt.2020.00315
Schennach R, Zill P, Obermeier M et al (2012) The CNR1 gene in depression and schizophrenia — Is there an association with early improvement and response? Psychiatry Res 196:160. https://doi.org/10.1016/j.psychres.2011.11.021
Domschke K, Dannlowski U, Ohrmann P et al (2008) Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur Neuropsychopharmacol 18:751–759. https://doi.org/10.1016/j.euroneuro.2008.05.003
Koethe D, Llenos IC, Dulay JR et al (2007) Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm 114:1055–1063. https://doi.org/10.1007/s00702-007-0660-5
Choi K, Le T, McGuire J et al (2012) Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J Psychiatr Res 46:882–889. https://doi.org/10.1016/j.jpsychires.2012.03.021
Kong X, Miao Q, Lu X et al (2019) The association of endocannabinoid receptor genes (CNR1 and CNR2) polymorphisms with depression: a meta-analysis. Medicine (Baltimore) 98:e17403. https://doi.org/10.1097/MD.0000000000017403
Ishiguro H, Horiuchi Y, Tabata K et al (2018) Cannabinoid CB2 receptor gene and environmental interaction in the development of psychiatric disorders. Molecules 23:1836. https://doi.org/10.3390/molecules23081836
García-Gutiérrez M, Pérez-Ortiz J, Gutiérrez-Adán A, Manzanares J (2010) Depression-resistant endophenotype in mice overexpressing cannabinoid CB2 receptors: depression and CB2r. Br J Pharmacol 160:1773–1784. https://doi.org/10.1111/j.1476-5381.2010.00819.x
Hill M, Miller G, Ho W-S et al (2008) Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry 41:48–53. https://doi.org/10.1055/s-2007-993211
Hill MN, Miller GE, Carrier EJ et al (2009) Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34:1257–1262. https://doi.org/10.1016/j.psyneuen.2009.03.013
Ranganathan M, Cortes-Briones J, Radhakrishnan R et al (2016) Reduced brain cannabinoid receptor availability in schizophrenia. Biol Psychiatry 79:997–1005. https://doi.org/10.1016/j.biopsych.2015.08.021
Potvin S, Mahrouche L, Assaf R et al (2020) Peripheral endogenous cannabinoid levels are increased in schizophrenia patients evaluated in a psychiatric emergency setting. Front Psychiatry 11:628
Rodríguez-Muñoz M, Sánchez-Blázquez P, Callado LF et al (2017) Schizophrenia and depression, two poles of endocannabinoid system deregulation. Transl Psychiatry 7:1291. https://doi.org/10.1038/s41398-017-0029-y
Romero-Sanchiz P, Nogueira-Arjona R, Mayoral-Cleríes F et al (2016) Plasma concentrations of endocannabinoids and congeners in a primary care sample of depressed patients: influence of biological variables, severity and antidepressant medication. Eur Psychiatry 33:S422–S423. https://doi.org/10.1016/j.eurpsy.2016.01.1524
Meyer JD, Crombie KM, Cook DB et al (2019) Serum endocannabinoid and mood changes after exercise in major depressive disorder. Med Sci Sports Exerc 51:1909–1917. https://doi.org/10.1249/MSS.0000000000002006
Kranaster L, Hoyer C, Aksay SS et al (2017) Electroconvulsive therapy enhances endocannabinoids in the cerebrospinal fluid of patients with major depression: a preliminary prospective study. Eur Arch Psychiatry Clin Neurosci 267:781–786. https://doi.org/10.1007/s00406-017-0789-7
Garani R, Watts JJ, Mizrahi R (2021) Endocannabinoid system in psychotic and mood disorders, a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry 106:110096. https://doi.org/10.1016/j.pnpbp.2020.110096
Minocci D, Massei J, Martino A et al (2011) Genetic association between bipolar disorder and 524A>C (Leu133Ile) polymorphism of CNR2 gene, encoding for CB2 cannabinoid receptor. J Affect Disord 134:427–430. https://doi.org/10.1016/j.jad.2011.05.023
Arjmand S, Behzadi M, Kohlmeier KA et al (2019) Bipolar disorder and the endocannabinoid system. Acta Neuropsychiatr 31:193–201. https://doi.org/10.1017/neu.2019.21
Leweke FM, Koethe D (2008) Cannabis and psychiatric disorders: it is not only addiction: Cannabis and psychiatric disorders. Addict Biol 13:264–275. https://doi.org/10.1111/j.1369-1600.2008.00106.x
Ashton CH, Moore PB, Gallagher P, Young AH (2005) Cannabinoids in bipolar affective disorder: a review and discussion of their therapeutic potential. J Psychopharmacol (Oxf) 19:293–300. https://doi.org/10.1177/0269881105051541
Lazary J, Eszlari N, Kriko E et al (2021) Genetic analyses of the endocannabinoid pathway in association with affective phenotypic variants. Neurosci Lett 744:135600. https://doi.org/10.1016/j.neulet.2020.135600
Liu WS, Soldatov NM, Gustavsson I, Chowdhary BP (1998) Fiber-FISH analysis of the 3’-terminal region of the human L-type Ca2+ channel alpha 1C subunit gene. Hereditas 129:169–175. https://doi.org/10.1111/j.1601-5223.1998.00169.x
Casamassima F, Hay AC, Benedetti A et al (2010) L-type calcium channels and psychiatric disorders: a brief review. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet 153B:1373–1390. https://doi.org/10.1002/ajmg.b.31122
Dickens AM, Borgan F, Laurikainen H et al (2020) Links between central CB1-receptor availability and peripheral endocannabinoids in patients with first episode psychosis. Npj Schizophr 6:21. https://doi.org/10.1038/s41537-020-00110-7
Wong DF, Kuwabara H, Horti AG et al (2010) Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage 52:1505–1513. https://doi.org/10.1016/j.neuroimage.2010.04.034
Volk DW, Eggan SM, Horti AG et al (2014) Reciprocal alterations in cortical cannabinoid receptor 1 binding relative to protein immunoreactivity and transcript levels in schizophrenia. Schizophr Res 159:124–129. https://doi.org/10.1016/j.schres.2014.07.017
Mihov Y (2016) Positron emission tomography studies on cannabinoid receptor type 1 in schizophrenia. Biol Psychiatry 79:e97–e99. https://doi.org/10.1016/j.biopsych.2016.04.015
Ceccarini J, De Hert M, Van Winkel R et al (2013) Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage 79:304–312. https://doi.org/10.1016/j.neuroimage.2013.04.052
Borgan F, Laurikainen H, Veronese M et al (2019) In vivo availability of cannabinoid 1 receptor levels in patients with first-episode psychosis. JAMA Psychiat 76:1074. https://doi.org/10.1001/jamapsychiatry.2019.1427
Borgan F, Veronese M, Reis Marques T et al (2021) Association between cannabinoid 1 receptor availability and glutamate levels in healthy controls and drug-free patients with first episode psychosis: a multi-modal PET and 1H-MRS study. Eur Arch Psychiatry Clin Neurosci 271:677–687. https://doi.org/10.1007/s00406-020-01191-2
Cortez IL, Rodrigues da Silva N, Guimarães FS, Gomes FV (2020) Are CB2 receptors a new target for schizophrenia treatment? Front Psychiatry 11:587154. https://doi.org/10.3389/fpsyt.2020.587154
Ishiguro H, Horiuchi Y, Ishikawa M et al (2010) Brain cannabinoid CB2 receptor in schizophrenia. Biol Psychiatry 67:974–982. https://doi.org/10.1016/j.biopsych.2009.09.024
Banaszkiewicz I, Biala G, Kruk-Slomka M (2020) Contribution of CB2 receptors in schizophrenia-related symptoms in various animal models: short review. Neurosci Biobehav Rev 114:158–171. https://doi.org/10.1016/j.neubiorev.2020.04.020
Minichino A, Senior M, Brondino N et al (2019) Measuring disturbance of the endocannabinoid system in psychosis: a systematic review and meta-analysis. JAMA Psychiat 76:914. https://doi.org/10.1001/jamapsychiatry.2019.0970
Joaquim HPG, Costa AC, Pereira CAC et al (2022) Plasmatic endocannabinoids are decreased in subjects with ultra-high risk of psychosis. Eur J Neurosci 55:1079–1087. https://doi.org/10.1111/ejn.15509
Laprairie RB, Bagher AM, Kelly MEM, Denovan-Wright EM (2015) Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor: negative allosteric modulation of CB 1 by cannabidiol. Br J Pharmacol 172:4790–4805. https://doi.org/10.1111/bph.13250
Martínez-Pinilla E, Varani K, Reyes-Resina I et al (2017) Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol 8:744. https://doi.org/10.3389/fphar.2017.00744
Bisogno T, Hanuš L, De Petrocellis L et al (2001) Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide: Cannabidiol, VR1 receptors and anandamide inactivation. Br J Pharmacol 134:845–852. https://doi.org/10.1038/sj.bjp.0704327
O’Sullivan SE (2016) An update on PPAR activation by cannabinoids: cannabinoids and PPARs. Br J Pharmacol 173:1899–1910. https://doi.org/10.1111/bph.13497
Giacoppo S, Pollastro F, Grassi G et al (2017) Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia 116:77–84. https://doi.org/10.1016/j.fitote.2016.11.010
Jastrząb A, GegotekSkrzydlewska AE (2019) Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells 8:827. https://doi.org/10.3390/cells8080827
Kozela E, Pietr M, Juknat A et al (2010) Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem 285:1616–1626. https://doi.org/10.1074/jbc.M109.069294
Singer E, Judkins J, Salomonis N et al (2015) Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis 6:e1601–e1601. https://doi.org/10.1038/cddis.2014.566
Juknat A, Pietr M, Kozela E et al (2012) Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Δ9-tetrahydrocannabinol in BV-2 microglial cells: Cannabinoids transcriptional profiles. Br J Pharmacol 165:2512–2528. https://doi.org/10.1111/j.1476-5381.2011.01461.x
Casares L, García V, Garrido-Rodríguez M et al (2020) Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol 28:101321. https://doi.org/10.1016/j.redox.2019.101321
Campos AC, Fogaça MV, Sonego AB, Guimarães FS (2016) Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 112:119–127. https://doi.org/10.1016/j.phrs.2016.01.033
Chen J, Hou C, Chen X et al (2016) Protective effect of cannabidiol on hydrogen peroxide-induced apoptosis, inflammation and oxidative stress in nucleus pulposus cells. Mol Med Rep 14:2321–2327. https://doi.org/10.3892/mmr.2016.5513
Rajan TS, Giacoppo S, Iori R et al (2016) Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 112:104–115. https://doi.org/10.1016/j.fitote.2016.05.008
Pan H, Mukhopadhyay P, Rajesh M et al (2009) Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther 328:708–714. https://doi.org/10.1124/jpet.108.147181
Esposito G, Scuderi C, Savani C et al (2007) Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression: CBD blunts Aβ induced neuroinflammation in vivo>. Br J Pharmacol 151:1272–1279. https://doi.org/10.1038/sj.bjp.0707337
Fouad AA, Jresat I (2012) Hepatoprotective effect of coenzyme Q10 in rats with acetaminophen toxicity. Environ Toxicol Pharmacol 33:158–167. https://doi.org/10.1016/j.etap.2011.12.011
Costa B, Trovato AE, Comelli F et al (2007) The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 556:75–83. https://doi.org/10.1016/j.ejphar.2006.11.006
Rajesh M, Mukhopadhyay P, Bátkai S et al (2007) Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol-Heart Circ Physiol 293:H610–H619. https://doi.org/10.1152/ajpheart.00236.2007
Martín-Hernández D, Caso JR, Javier Meana J et al (2018) Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: effect of antidepressants. J Neuroinflammation 15:251. https://doi.org/10.1186/s12974-018-1294-2
Zhang J, Yao W, Dong C et al (2018) Keap1–Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci 268:865–870. https://doi.org/10.1007/s00406-017-0848-0
Genc K, Genc S (2009) Oxidative stress and dysregulated Nrf2 activation in the pathogenesis of schizophrenia. Biosci Hypotheses 2:16–18. https://doi.org/10.1016/j.bihy.2008.10.005
Colle R, de Larminat D, Rotenberg S et al (2016) PPAR-γ agonists for the treatment of major depression: a review. Pharmacopsychiatry 50:49–55. https://doi.org/10.1055/s-0042-120120
Nierenberg AA, Ghaznavi SA, Sande Mathias I et al (2018) Peroxisome proliferator-activated receptor gamma coactivator-1 alpha as a novel target for bipolar disorder and other neuropsychiatric disorders. Biol Psychiatry 83:761–769. https://doi.org/10.1016/j.biopsych.2017.12.014
García-Bueno B, Bioque M, Mac-Dowell KS et al (2014) Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull 40:376–387. https://doi.org/10.1093/schbul/sbt001
Koo JW, Russo SJ, Ferguson D et al (2010) Nuclear factor-B is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci 107:2669–2674. https://doi.org/10.1073/pnas.0910658107
Elhaik E, Zandi P (2015) Dysregulation of the NF-κB pathway as a potential inducer of bipolar disorder. J Psychiatr Res 70:18–27. https://doi.org/10.1016/j.jpsychires.2015.08.009
Volk DW, Moroco AE, Roman KM et al (2019) The role of the nuclear factor-κB transcriptional complex in cortical immune activation in schizophrenia. Biol Psychiatry 85:25–34. https://doi.org/10.1016/j.biopsych.2018.06.015
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca2+ signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct 32:134–141. https://doi.org/10.3109/10799893.2012.672994
Ruggiero RN, Rossignoli MT, De Ross JB et al (2017) Cannabinoids and vanilloids in schizophrenia: neurophysiological evidence and directions for basic research. Front Pharmacol 8:399. https://doi.org/10.3389/fphar.2017.00399
Muller C, Morales P, Reggio PH (2019) Cannabinoid ligands targeting TRP channels. Front Mol Neurosci 11:487. https://doi.org/10.3389/fnmol.2018.00487
Staurengo-Ferrari L, Badaro-Garcia S, Hohmann MSN et al (2019) Contribution of Nrf2 modulation to the mechanism of action of analgesic and anti-inflammatory drugs in pre-clinical and clinical stages. Front Pharmacol 9:1536. https://doi.org/10.3389/fphar.2018.01536
van Horssen J, Drexhage JAR, Flor T et al (2010) Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic Biol Med 49:1283–1289. https://doi.org/10.1016/j.freeradbiomed.2010.07.013
Schulze-Topphoff U, Varrin-Doyer M, Pekarek K et al (2016) Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci 113:4777–4782. https://doi.org/10.1073/pnas.1603907113
Mills EA, Ogrodnik MA, Plave A, Mao-Draayer Y (2018) Emerging understanding of the mechanism of action for dimethyl fumarate in the treatment of multiple sclerosis. Front Neurol 9:5. https://doi.org/10.3389/fneur.2018.00005
Hammer A, Waschbisch A, Kuhbandner K et al (2018) The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis. Ann Clin Transl Neurol 5:668–676. https://doi.org/10.1002/acn3.553
Gopal S, Mikulskis A, Gold R et al (2017) Evidence of activation of the Nrf2 pathway in multiple sclerosis patients treated with delayed-release dimethyl fumarate in the Phase 3 DEFINE and CONFIRM studies. Mult Scler J 23:1875–1883. https://doi.org/10.1177/1352458517690617
Kourakis S, Timpani CA, de Haan JB et al (2020) Dimethyl fumarate and its esters: a drug with broad clinical utility? Pharmaceuticals 13:306. https://doi.org/10.3390/ph13100306
Albrecht P, Bouchachia I, Goebels N et al (2012) Effects of dimethyl fumarate on neuroprotection and immunomodulation. J Neuroinflammation 9:163. https://doi.org/10.1186/1742-2094-9-163
Gill AJ, Kolson DL (2013) Dimethyl fumarate modulation of immune and antioxidant responses: application to HIV therapy. Crit Rev Immunol 33:307–359. https://doi.org/10.1615/CritRevImmunol.2013007247
Huang H, Taraboletti A, Shriver LP (2015) Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol 5:169–175. https://doi.org/10.1016/j.redox.2015.04.011
Fox RJ, Miller DH, Phillips JT et al (2012) Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 367:1087–1097. https://doi.org/10.1056/NEJMoa1206328
Gold PW, Pavlatou MG, Carlson PJ et al (2012) Unmedicated, remitted patients with major depression have decreased serum immunoglobulin A. Neurosci Lett 520:1–5. https://doi.org/10.1016/j.neulet.2012.04.072
Kappos L, Antel J, Comi G et al (2006) Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 355:1124–1140. https://doi.org/10.1056/NEJMoa052643
Gold R, Giovannoni G, Phillips JT et al (2015) Efficacy and safety of delayed-release dimethyl fumarate in patients newly diagnosed with relapsing–remitting multiple sclerosis (RRMS). Mult Scler J 21:57–66. https://doi.org/10.1177/1352458514537013
Kappos L, Giovannoni G, Gold R et al (2015) Time course of clinical and neuroradiological effects of delayed-release dimethyl fumarate in multiple sclerosis. Eur J Neurol 22:664–671. https://doi.org/10.1111/ene.12624
Gold R, Kappos L, Arnold DL et al (2012) Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 367:1098–1107. https://doi.org/10.1056/NEJMoa1114287
Gasim M, Bernstein CN, Graff LA et al (2018) Adverse psychiatric effects of disease-modifying therapies in multiple Sclerosis: a systematic review. Mult Scler Relat Disord 26:124–156. https://doi.org/10.1016/j.msard.2018.09.008
Acknowledgements
Michael Berk is supported by a NHMRC Senior Principal Research Fellowship. Wolfgang Marx is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia early-career fellowship.
Author information
Authors and Affiliations
Contributions
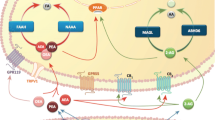
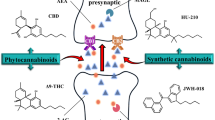
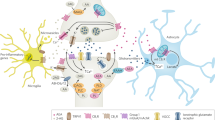
All the authors contributed to the construction and editing of the paper. The first draft was produced by GM and the final draft by BKP. The revised version was produced by BKP. Figure 1 was produced by BKP and Fig. 2 was produced jointly by GM and BKP.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Not applicable.
Competing interests
Michael Berk has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck and Merck and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier – all unrelated to this work.
Wolfgang Marx is currently funded by an Alfred Deakin Postdoctoral Research Fellowship and a Multiple Sclerosis Research Australia early-career fellowship. Wolfgang has previously received funding from the NHMRC, Clifford Craig Foundation, Cancer Council Queensland and university grants/fellowships from La Trobe University, Deakin University, University of Queensland, and Bond University, received industry funding and has attended events funded by Cobram Estate Pty. Ltd, received travel funding from Nutrition Society of Australia, received consultancy funding from Nutrition Research Australia, and has received speakers honoraria from The Cancer Council Queensland and the Princess Alexandra Research Foundation.
None of the other authors has any competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morris, G., Sominsky, L., Walder, K.R. et al. Inflammation and Nitro-oxidative Stress as Drivers of Endocannabinoid System Aberrations in Mood Disorders and Schizophrenia. Mol Neurobiol 59, 3485–3503 (2022). https://doi.org/10.1007/s12035-022-02800-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02800-y