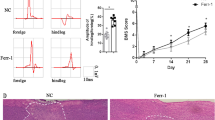
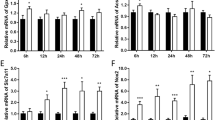
Abstract
Spinal cord injury (SCI), a devastating neurological impairment, usually imposes a long-term psychological stress and high socioeconomic burden for the sufferers and their family. Recent researchers have paid arousing attention to white matter injury and the underlying mechanism following SCI. Ferroptosis has been revealed to be associated with diverse diseases including stroke, cancer, and kidney degeneration. Ferrostatin-1, a potent inhibitor of ferroptosis, has been illustrated to curb ferroptosis in neurons, subsequently improving functional recovery after traumatic brain injury (TBI) and SCI. However, the role of ferroptosis in white matter injury and the therapeutic effect of ferrostatin-1 on SCI are still unknown. Here, our results indicated that ferroptosis played a pivotal role in the secondary white matter injury, and ferrostatin-1 could reduce iron and reactive oxygen species (ROS) accumulation and downregulate the ferroptosis-related genes and its products of IREB2 and PTGS2 to further inhibit ferroptosis in oligodendrocyte, finally reducing white matter injury and promoting functional recovery following SCI in rats. Meanwhile, the results demonstrated that ferrostatin-1 held the potential of inhibiting the activation of reactive astrocyte and microglia. Mechanically, the present study deciphers the potential mechanism of white matter damage, which enlarges the therapeutic effects of ferrostatin-1 on SCI and even in other central nervous system (CNS) diseases existing ferroptosis.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- SCI:
-
Spinal cord injury
- TBI:
-
Traumatic brain injury
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system
- ASIC 1a:
-
Acid-sensing ion channel 1a
- GPER1:
-
G-protein coupled estrogen receptor 1
- NSCs:
-
Neural stem cells
- DFX:
-
Deferoxamine
- DMT1:
-
Divalent metal transporter 1
- RCD:
-
Regulated cell death
- BBB:
-
Blood–brain barrier
- OPCs:
-
Oligodendrocyte progenitor cells
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- PDGF:
-
Platelet-derived growth factor
- PBS:
-
Phosphate-buffered saline
- IHC:
-
Immunohistochemistry
- DAB:
-
3-Diaminobenzidine
- BCA:
-
Bicinchoninic acid
- HRP:
-
Horseradish peroxidase
- RT-qPCR:
-
Reverse transcription-quantitative polymerase chain reaction
- TEM:
-
Transmission electron microscopy
References
Hutson TH, Di Giovanni S (2019) The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat Rev Neurol 15(12):732–745. https://doi.org/10.1038/s41582-019-0280-3
Hu R, Sun H, Zhang Q, Chen J, Wu N, Meng H, Cui G, Hu S, Li F, Lin J, Wan Q, Feng H (2012) G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit Care Med 40(12):3230–3237. https://doi.org/10.1097/CCM.0b013e3182657560
Hu SL, Lu PG, Zhang LJ, Li F, Chen Z, Wu N, Meng H, Lin JK, Feng H (2012) In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J Cell Biochem 113(3):1005–1012. https://doi.org/10.1002/jcb.23432
Hu R, Duan B, Wang D, Yu Y, Li W, Luo H, Lu P, Lin J, Zhu G, Wan Q, Feng H (2011) Role of acid-sensing ion channel 1a in the secondary damage of traumatic spinal cord injury. Ann Surg 254(2):353–362. https://doi.org/10.1097/SLA.0b013e31822645b4
Chen J, Hu R, Ge H, Duanmu W, Li Y, Xue X, Hu S, Feng H (2015) G-protein-coupled receptor 30-mediated antiapoptotic effect of estrogen on spinal motor neurons following injury and its underlying mechanisms. Mol Med Rep 12(2):1733–1740. https://doi.org/10.3892/mmr.2015.3601
Li L, Xiong ZY, Qian ZM, Zhao TZ, Feng H, Hu S, Hu R, Ke Y, Lin J (2014) Complement C5a is detrimental to histological and functional locomotor recovery after spinal cord injury in mice. Neurobiol Dis 66:74–82. https://doi.org/10.1016/j.nbd.2014.02.008
Yuan J, Liu W, Zhu H, Chen Y, Zhang X, Li L, Chu W, Wen Z, Feng H, Lin J (2017) Curcumin inhibits glial scar formation by suppressing astrocyte-induced inflammation and fibrosis in vitro and in vivo. Brain Res 1655:90–103. https://doi.org/10.1016/j.brainres.2016.11.002
Xia Y, Zhao T, Li J, Li L, Hu R, Hu S, Feng H, Lin J (2008) Antisense vimentin cDNA combined with chondroitinase ABC reduces glial scar and cystic cavity formation following spinal cord injury in rats. Biochem Biophys Res Commun 377(2):562–566. https://doi.org/10.1016/j.bbrc.2008.10.024
Xia Y, Yan Y, Xia H, Zhao T, Chu W, Hu S, Feng H, Lin J (2015) Antisense vimentin cDNA combined with chondroitinase ABC promotes axon regeneration and functional recovery following spinal cord injury in rats. Neurosci Lett 590:74–79. https://doi.org/10.1016/j.neulet.2015.01.073
Hu SL, Luo HS, Li JT, Xia YZ, Li L, Zhang LJ, Meng H, Cui GY, Chen Z, Wu N, Lin JK, Zhu G, Feng H (2010) Functional recovery in acute traumatic spinal cord injury after transplantation of human umbilical cord mesenchymal stem cells. Crit Care Med 38(11):2181–2189. https://doi.org/10.1097/CCM.0b013e3181f17c0e
Chu W, Yuan J, Huang L, Xiang X, Zhu H, Chen F, Chen Y, Lin J, Feng H (2015) Valproic acid arrests proliferation but promotes neuronal differentiation of adult spinal NSPCs from SCI rats. Neurochem Res 40(7):1472–1486. https://doi.org/10.1007/s11064-015-1618-x
Chen F, Wang H, Xiang X, Yuan J, Chu W, Xue X, Zhu H, Ge H, Zou M, Feng H, Lin J (2014) Curcumin increased the differentiation rate of neurons in neural stem cells via wnt signaling in vitro study. J Surg Res 192(2):298–304. https://doi.org/10.1016/j.jss.2014.06.026
Mekhail M, Almazan G, Tabrizian M (2012) Oligodendrocyte-protection and remyelination post-spinal cord injuries: a review. Prog Neurobiol 96(3):322–339. https://doi.org/10.1016/j.pneurobio.2012.01.008
Fehlings MG, Tator CH (1995) The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol 132(2):220–228. https://doi.org/10.1016/0014-4886(95)90027-6
Kakulas BA (1999) A review of the neuropathology of human spinal cord injury with emphasis on special features. J Spinal Cord Med 22(2):119–124. https://doi.org/10.1080/10790268.1999.11719557
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81(2):871–927. https://doi.org/10.1152/physrev.2001.81.2.871
Juurlink BH, Thorburne SK, Hertz L (1998) Peroxide-scavenging deficit underlies oligodendrocyte susceptibility to oxidative stress. Glia 22(4):371–378. https://doi.org/10.1002/(sici)1098-1136(199804)22:4%3c371::aid-glia6%3e3.0.co;2-6
Thorburne SK, Juurlink BH (1996) Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem 67(3):1014–1022. https://doi.org/10.1046/j.1471-4159.1996.67031014.x
Fan BY, Pang YL, Li WX, Zhao CX, Zhang Y, Wang X, Ning GZ, Kong XH, Liu C, Yao X, Feng SQ (2021) Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen Res 16(3):561–566. https://doi.org/10.4103/1673-5374.293157
Shi J, Tang R, Zhou Y, Xian J, Zuo C, Wang L, Wang J, Feng H, Hu S (2020) Attenuation of white matter damage following deferoxamine treatment in rats after spinal cord injury. World Neurosurg 137:e9–e17. https://doi.org/10.1016/j.wneu.2019.08.246
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Feng Z, Min L, Chen H, Deng W, Tan M, Liu H, Hou J (2021) Iron overload in the motor cortex induces neuronal ferroptosis following spinal cord injury. Redox Biol 43:101984. https://doi.org/10.1016/j.redox.2021.101984
Chu J, Liu CX, Song R, Li QL (2020) Ferrostatin-1 protects HT-22 cells from oxidative toxicity. Neural Regen Res 15(3):528–536. https://doi.org/10.4103/1673-5374.266060
Miotto G, Rossetto M, Di Paolo ML, Orian L, Venerando R, Roveri A, Vučković AM, Bosello Travain V, Zaccarin M, Zennaro L, Maiorino M, Toppo S, Ursini F, Cozza G (2020) Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol 28:101328. https://doi.org/10.1016/j.redox.2019.101328
Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR (2018) Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci 12:466. https://doi.org/10.3389/fnins.2018.00466
Li S, Zhou C, Zhu Y, Chao Z, Sheng Z, Zhang Y, Zhao Y (2020) Ferrostatin-1 alleviates angiotensin II (Ang II)- induced inflammation and ferroptosis in astrocytes. Int Immunopharmacol 90:107179. https://doi.org/10.1016/j.intimp.2020.107179
Lu J, Xu F, Lu H (2020) LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci 260:118305. https://doi.org/10.1016/j.lfs.2020.118305
Shen L, Lin D, Li X, Wu H, Lenahan C, Pan Y, Xu W, Chen Y, Shao A, Zhang J (2020) Ferroptosis in acute central nervous system injuries: the future direction? Front Cell Dev Biol 8:594. https://doi.org/10.3389/fcell.2020.00594
Chen B, Chen Z, Liu M, Gao X, Cheng Y, Wei Y, Wu Z, Cui D, Shang H (2019) Inhibition of neuronal ferroptosis in the acute phase of intracerebral hemorrhage shows long-term cerebroprotective effects. Brain Res Bull 153:122–132. https://doi.org/10.1016/j.brainresbull.2019.08.013
Li Y, Liu Y, Wu P, Tian Y, Liu B, Wang J, Bihl J, Shi H (2021) Inhibition of ferroptosis alleviates early brain injury after subarachnoid hemorrhage in vitro and in vivo via reduction of lipid peroxidation. Cell Mol Neurobiol 41(2):263–278. https://doi.org/10.1007/s10571-020-00850-1
Xie BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, Jiang JY (2019) Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther 25(4):465–475. https://doi.org/10.1111/cns.13069
Chen X, Gao C, Yan Y, Cheng Z, Chen G, Rui T, Luo C, Gao Y, Wang T, Chen X, Tao L (2021) Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Exp Neurol 342:113762. https://doi.org/10.1016/j.expneurol.2021.113762
Qiu Y, Cao Y, Cao W, Jia Y, Lu N (2020) The application of ferroptosis in diseases. Pharmacol Res 159:104919. https://doi.org/10.1016/j.phrs.2020.104919
Yumnamcha T, Devi TS, Singh LP (2019) Auranofin mediates mitochondrial dysregulation and inflammatory cell death in human retinal pigment epithelial cells: implications of retinal neurodegenerative diseases. Front Neurosci 13:1065. https://doi.org/10.3389/fnins.2019.01065
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21. https://doi.org/10.1089/neu.1995.12.1
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139(2):244–256. https://doi.org/10.1006/exnr.1996.0098
Serdar M, Mordelt A, Müser K, Kempe K, Felderhoff-Müser U, Herz J, Bendix I (2019) Detrimental impact of energy drink compounds on developing oligodendrocytes and neurons. Cells. https://doi.org/10.3390/cells8111381
Han S, Tang Q, Chen R, Li Y, Shu J, Zhang X (2017) Hepatic iron overload is associated with hepatocyte apoptosis during Clonorchis sinensis infection. BMC Infect Dis 17(1):531. https://doi.org/10.1186/s12879-017-2630-3
Jiang X, Zhang J, Kou B, Zhang C, Zhong J, Fang X, Huang X, Zhang X, Xie F, Hu Q, Ge H, Yu A (2020) Ambroxol improves neuronal survival and reduces white matter damage through suppressing endoplasmic reticulum stress in microglia after intracerebral hemorrhage. Biomed Res Int 2020:8131286. https://doi.org/10.1155/2020/8131286
Muhoberac BB, Vidal R (2019) Iron, ferritin, hereditary ferritinopathy, and neurodegeneration. Front Neurosci 13:1195. https://doi.org/10.3389/fnins.2019.01195
Bin JM, Harris SN, Kennedy TE (2016) The oligodendrocyte-specific antibody “CC1” binds quaking 7. J Neurochem 139(2):181–186. https://doi.org/10.1111/jnc.13745
Boggs JM (2006) Myelin basic protein: a multifunctional protein. Cell Mol Life Sci 63(17):1945–1961. https://doi.org/10.1007/s00018-006-6094-7
Guo Y, Du J, Xiao C, Xiang P, Deng Y, Hei Z, Li X (2021) Inhibition of ferroptosis-like cell death attenuates neuropathic pain reactions induced by peripheral nerve injury in rats. Eur J Pain (London, England) 25(6):1227–1240. https://doi.org/10.1002/ejp.1737
Ling X, Liu D (2007) Temporal and spatial profiles of cell loss after spinal cord injury: reduction by a metalloporphyrin. J Neurosci Res 85(10):2175–2185. https://doi.org/10.1002/jnr.21362
Ek CJ, Habgood MD, Callaway JK, Dennis R, Dziegielewska KM, Johansson PA, Potter A, Wheaton B, Saunders NR (2010) Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PLoS ONE 5(8):e12021. https://doi.org/10.1371/journal.pone.0012021
Zhang Y, Fan BY, Pang YL, Shen WY, Wang X, Zhao CX, Li WX, Liu C, Kong XH, Ning GZ, Feng SQ, Yao X (2020) Neuroprotective effect of deferoxamine on erastininduced ferroptosis in primary cortical neurons. Neural Regen Res 15(8):1539–1545. https://doi.org/10.4103/1673-5374.274344
Wang J, Chen Y, Chen L, Duan Y, Kuang X, Peng Z, Li C, Li Y, Xiao Y, Jin H, Tan Q, Zhang S, Zhu B, Tang Y (2020) EGCG modulates PKD1 and ferroptosis to promote recovery in ST rats. Transl Neurosci 11(1):173–181. https://doi.org/10.1515/tnsci-2020-0119
Ge MH, Tian H, Mao L, Li DY, Lin JQ, Hu HS, Huang SC, Zhang CJ, Mei XF (2021) Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther 27(9):1023–1040. https://doi.org/10.1111/cns.13657
Guerrero-Hue M, García-Caballero C, Palomino-Antolín A, Rubio-Navarro A, Vázquez-Carballo C, Herencia C, Martín-Sanchez D, Farré-Alins V, Egea J, Cannata P, Praga M, Ortiz A, Egido J, Sanz AB, Moreno JA (2019) Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J 33(8):8961–8975. https://doi.org/10.1096/fj.201900077R
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (approval no. 81471261) and Natural Science Foundation of Chongqing (approval no. cstc2018jcyjAX0080).
Author information
Authors and Affiliations
Contributions
HFG and XSX performed most of the experiments, with assistance from JSX, LBY, YJZ, JZ, JTS, LW, ZYJ, HS, and TNC. HFG and XSX analyzed the results and edited figures. JSX, YJZ, ZYJ, and JZ performed SCI model and statistical analysis. HFG and LW performed cell culture and treatments. HFG, JTS, JW, and TNC performed immunoblotting and immunostaining. HFG wrote preliminary draft of the manuscript. SLH and HF designed experiments and revised the manuscript. All authors approved final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Informed Consent
Not applicable.
Research Involving Human and Animals Participants
All experiments were conducted in accordance with the China’s animal welfare legislation for the protection of animals used for scientific purposes. And all procedures were supervised by the Ethics Committee of the Southwest Hospital, Third Military Medical University for the use of laboratory animals (approval no. SYXK 20170002).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, H., Xue, X., Xian, J. et al. Ferrostatin-1 Alleviates White Matter Injury Via Decreasing Ferroptosis Following Spinal Cord Injury. Mol Neurobiol 59, 161–176 (2022). https://doi.org/10.1007/s12035-021-02571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02571-y