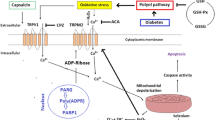
Abstract
Neuropathic pain and oxidative neurotoxicity are two adverse main actions of diabetes mellitus (DM). The expression levels of calcium ion (Ca2+) permeable TRPV1 channels are high in the dorsal root ganglion (DRGs) and hippocampus (HIPPO). TRPV1 is activated by capsaicin and reactive free oxygen radicals (fROS) to mediate peripheral neuropathy and neurotoxicity. Noopept (NP) acted several protective antioxidant actions against oxidative neurotoxicity. As DM is known to increase the levels of fROS, the protective roles of antioxidant NP were evaluated on the DM-mediated neurotoxicity and neuropathic pain via the modulation of TRPV1 in rats. Thirty-six rats were equally divided into control, NP, DM (streptozotocin, STZ), and STZ + NP groups. A decrease on the STZ-mediated increase of neuropathic pain (via the analyses of Von Frey and hot plate) and blood glucose level was observed by the treatment of NP. A protective role of NP via downregulation of TRPV1 activity on the STZ-induced increase of apoptosis, mitochondrial fROS, lipid peroxidation, caspase -3 (CASP-3), caspase -9 (CASP-9), TRPV1 current density, glutathione (GSH), cytosolic free Zn2+, and Ca2+ concentrations in the DRGs and HIPPO was also observed. The STZ-mediated decrease of glutathione peroxidase, GSH, vitamin E, and β-carotene concentrations in the brain cortex, erythrocyte, liver, kidney, and plasma was also attenuated by the treatment of NP. The STZ-mediated increase of TRPV1, CASP-3, and CASP-9 expressions was decreased in the DRGs and HIPPO by the treatment of NP. In conclusion, the treatment of NP induced protective effects against STZ-induced adverse peripheral pain and HIPPO oxidative neurotoxicity. These effects might attribute to the potent antioxidant property of NP.











Similar content being viewed by others
Availability of Data and Materials
The row data are available from the Professor M. Nazıroğlu on reasonable request. The preparation of the graphics in the manuscript were performed by Professor M. Nazıroğlu.
Abbreviations
- DM:
-
Diabetes mellitus
- DNP:
-
Diabetic neuropathy
- DRGs:
-
Dorsal root ganglions
- HIPPO:
-
Hippocampus
- Int-fROS:
-
Intracellular fROS
- NP:
-
Noopept
- fROS:
-
Free reactive oxygen radicals
- STZ:
-
Streptozotocin
- TRPV1:
-
Transient receptor vanilloid 1
- mPTP:
-
Mitochondrial permeability transition pore
References
Fathizadeh H, Milajerdi A, Reiner Ž et al (2020) The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Metab Disord 19(2):1879–1894. https://doi.org/10.1007/s40200-020-00627-9
Naranjo C, Ortega-Jiménez P, Del Reguero L, Moratalla G, Failde I (2020) Relationship between diabetic neuropathic pain and comorbidity. Their impact on pain intensity, diabetes complications and quality of life in patients with type-2 diabetes mellitus. Diabetes Res Clin Pract 165:108236. https://doi.org/10.1016/j.diabres.2020.108236
Basu P, Basu A (2020) In vitro and in vivo effects of flavonoids on peripheral neuropathic pain. Molecules 25(5):1171. https://doi.org/10.3390/molecules25051171
Sima AA, Zhang W (2014) Mechanisms of diabetic neuropathy: axon dysfunction. Handb Clin Neurol 126:429–442. https://doi.org/10.1016/B978-0-444-53480-4.00031-X
Liu W, Liang XC, Shi Y (2020) Effects of hirudin on high glucose-induced oxidative stress and inflammatory pathway in rat dorsal root ganglion neurons. Chin J Integr Med 26(3):197–204. https://doi.org/10.1007/s11655-019-2712-8
Huerta-Cervantes M, Peña-Montes DJ, Montoya-Pérez R et al (2020) Gestational diabetes triggers oxidative stress in hippocampus and cerebral cortex and cognitive behavior modifications in rat offspring: Age- and sex-dependent effects. Nutrients 12(2):376. https://doi.org/10.3390/nu12020376
Carrasco C, Naziroǧlu M, Rodríguez AB, Pariente JA (2018) Neuropathic Pain: Delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol 9:95. https://doi.org/10.3389/fphys.2018.00095
Sözbir E, Nazıroğlu M (2016) Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab Brain Dis 31(2):385–393. https://doi.org/10.1007/s11011-015-9769-7
Özdemir ÜS, Nazıroğlu M, Şenol N, Ghazizadeh V (2016) Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: Involvement of TRPM2 and TRPV1 channels. Mol Neurobiol 53(6):3540–3551. https://doi.org/10.1007/s12035-015-9292-1
Garcilazo C, Cavallasca JA, Musuruana JL (2010) Shoulder manifestations of diabetes mellitus. Curr Diabetes Rev 6(5):334–340. https://doi.org/10.2174/157339910793360824
Knezevic NN, Jovanovic F, Candido KD, Knezevic I (2020) Oral pharmacotherapeutics for the management of peripheral neuropathic pain conditions - a review of clinical trials. Expert Opin Pharmacother 21(18):2231–2248. https://doi.org/10.1080/14656566.2020.1801635
Düll MM, Riegel K, Tappenbeck J, Ries V, Strupf M, Fleming T, Sauer SK, Namer B (2019) Methylglyoxal causes pain and hyperalgesia in human through C-fiber activation. Pain 160(11):2497–2507. https://doi.org/10.1097/j.pain.0000000000001644
Kahya MC, Nazıroğlu M, Övey İS (2017) Modulation of diabetes-induced oxidative stress, apoptosis, and Ca(2+) entry through TRPM2 and TRPV1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol Neurobiol 54(3):2345–2360. https://doi.org/10.1007/s12035-016-9727-3
Nazıroğlu M, Öz A, Yıldızhan K (2020) Selenium and neurological diseases: Focus on peripheral pain and TRP channels. Curr Neuropharmacol 18(6):501–517. https://doi.org/10.2174/1570159X18666200106152631
Nazıroğlu M, Dikici DM, Dursun S (2012) Role of oxidative stress and Ca2+ signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem Res 37(10):2065–2075. https://doi.org/10.1007/s11064-012-0850-x
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J Recept Signal Transduct Res 32(3):134–141. https://doi.org/10.3109/10799893.2012.672994
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Ibi M, Matsuno K, Shiba D et al (2008) Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci 28(38):9486–9494
Gavva NR, Tamir R, Qu Y et al (2005) AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther 313(1):474–484. https://doi.org/10.1124/jpet.104.079855
Yu L, Yang F, Luo H et al (2008) The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain 4:61. https://doi.org/10.1186/1744-8069-4-61
Hakimizadeh E, Oryan S, Hajizadeh Moghaddam A, Shamsizadeh A, Roohbakhsh A (2012) Endocannabinoid system and TRPV1 receptors in the dorsal hippocampus of the rats modulate anxiety-like behaviors. Iran J Basic Med Sci 15(3):795–802
Chukyo A, Chiba T, Kambe T et al (2018) Oxaliplatin-induced changes in expression of transient receptor potential channels in the dorsal root ganglion as a neuropathic mechanism for cold hypersensitivity. Neuropeptides 67:95–101. https://doi.org/10.1016/j.npep.2017.12.002
Tóth A, Boczán J, Kedei N et al (2005) Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res 135(1–2):162–168. https://doi.org/10.1016/j.molbrainres.2004.12.003
Kolbaev SN, Aleksandrova OP, Sharonova IN, Skrebitsky VG (2018) Effect of noopept on dynamics of intracellular calcium in neurons of cultured rat hippocampal slices. Bull Exp Biol Med 164(3):330–333. https://doi.org/10.1007/s10517-018-3983-3
Gürbüz P, Düzova H, Yildiz A et al (2019) Effects of noopept on cognitive functions and pubertal process in rats with diabetes. Life Sci 233:116698. https://doi.org/10.1016/j.lfs.2019.116698
Pelsman A, Hoyo-Vadillo C, Gudasheva TA, Seredenin SB, Ostrovskaya RU, Busciglio J (2003) GVS-111 prevents oxidative damage and apoptosis in normal and Down’s syndrome human cortical neurons. Int J Dev Neurosci 21(3):117–124. https://doi.org/10.1016/s0736-5748(03)00031-5
Wilms W, Woźniak-Karczewska M, Corvini PF, Chrzanowski Ł (2019) Nootropic drugs: Methylphenidate, modafinil and piracetam - Population use trends, occurrence in the environment, ecotoxicity and removal methods - A review. Chemosphere 233:771–785. https://doi.org/10.1016/j.chemosphere.2019.06.016
Nistor M, Behringer W, Schmidt M, Schiffner R (2017) A Systematic review of neuroprotective strategies during hypovolemia and hemorrhagic shock. Int J Mol Sci 18(11):2247. https://doi.org/10.3390/ijms18112247
Lutsenko VK, Vukolova MN, Gudasheva TA (2003) Cyclopropyl glycine and proline-containing preparation noopept evoke two types of membrane potential responses in synaptoneurosomes. Bull Exp Biol Med 135(6):559–562. https://doi.org/10.1023/a:1025425218023
Bukanova JV, Solntseva EI, Skrebitsky VG (2002) Selective suppression of the slow-inactivating potassium currents by nootropics in molluscan neurons. Int J Neuropsychopharmacol 5(3):229–237. https://doi.org/10.1017/S1461145702002997
Solntseva EI, Bukanova JV, Ostrovskaya RU, Gudasheva TA, Voronina TA, Skrebitsky VG (1997) The effects of piracetam and its novel peptide analogue GVS-111 on neuronal voltage-gated calcium and potassium channels. Gen Pharmacol 29(1):85–89. https://doi.org/10.1016/s0306-3623(96)00529-0
Andreeva NA, Stel’mashuk EV, Isaev NK, Ostrovskaya RU, Gudasheva TA, Viktorov IV (2000) Neuroprotective properties of nootropic dipeptide GVS-111 in in vitro oxygen-glucose deprivation, glutamate toxicity and oxidative stress. Bull Exp Biol Med 130(10):969–972
Ostrovskaya RU, Vakhitova YV, Kuzmina USh et al (2014) Neuroprotective effect of novel cognitive enhancer noopept on AD-related cellular model involves the attenuation of apoptosis and tau hyperphosphorylation. J Biomed Sci 21(1):74. https://doi.org/10.1186/s12929-014-0074-2
Ostrovskaya RU, Ozerova IV, Gudascheva TA, Kapitsa IG, Ivanova EA, Voronina TA, Seredenin SB (2013) Efficiency of noopept in streptozotocin-induced diabetes in rats. Bull Exp Biol Med 154(3):334–338. https://doi.org/10.1007/s10517-013-1944-4
Ostrovskaya RU, Zolotov NN, Ozerova IV, Ivanova EA, Kapitsa IG, Taraban KV, Michunskaya AM, Voronina TA et al (2014) Noopept normalizes parameters of the incretin system in rats with experimental diabetes. Bull Exp Biol Med 157(3):344–349. https://doi.org/10.1007/s10517-014-2562-5
Özkaya D, Nazıroğlu M, Armağan A et al (2011) Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem Funct 29(4):287–293. https://doi.org/10.1002/cbf.1749
Yüksel E, Nazıroğlu M, Şahin M, Çiğ B (2017) Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium. Sci Rep 7(1):17543. https://doi.org/10.1038/s41598-017-17715-1
Mohammadi-Farani A, Ghazi-Khansari M, Sahebgharani M (2014) Glucose concentration in culture medium affects mRNA expression of TRPV1 and CB1 receptors and changes capsaicin toxicity in PC12 cells. Iran J Basic Med Sci 17(9):673–378
Joshi DC, Bakowska JC (2011) Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp 51:2704. https://doi.org/10.3791/2704
Keil VC, Funke F, Zeug A, Schild D, Müller M (2011) Ratiometric high-resolution imaging of JC-1 fluorescence reveals the subcellular heterogeneity of astrocytic mitochondria. Pflugers Arch 462:693–708. https://doi.org/10.1007/s00424-011-1012-8
Yıldızhan K, Nazıroğlu M (2020) Glutathione depletion and parkinsonian neurotoxin MPP+-induced TRPM2 channel activation play central roles in oxidative cytotoxicity and inflammation in microglia. Mol Neurobiol 57(8):3508–3525. https://doi.org/10.1007/s12035-020-01974-7
Placer ZA, Cushman L, Johnson BC (1966) Estimation of products of lipid peroxidation (malonyl dialdehyde) in biological fluids. Anal Biochem 16:359–364
Sedlak J, Lindsay RHC (1968) Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmann’ s reagent. Anal Biochem 25:192–205
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Lawrence RA, Burk RF (2012) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 425(3):503–509
Desai ID (1980) Vitamin E analysis methods for animal tissues. Methods Enzymol 105:138–147
Suzuki J, Katoh N (1990) A simple and cheap method for measuring vitamin A in cattle using only a spectrophotometer. Jpn J Vet Sci 52:1282–1284
Racay P, Tatarkova Z, Chomova M, Hatok J, Kaplan P, Dobrota D (2009) Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus. Neurochem Res 34(8):1469–1478. https://doi.org/10.1007/s11064-009-9934-7
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R (2008) Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27(50):6407–6418. https://doi.org/10.1038/onc.2008.308
Yoon H, Thakur V, Isham D, Fayad M, Chattopadhyay M (2015) Moderate exercise training attenuates inflammatory mediators in DRG of Type 1 diabetic rats. Exp Neurol 267:107–114. https://doi.org/10.1016/j.expneurol.2015.03.006
Zhang BY, Zhang YL, Sun Q et al (2020) Alpha-lipoic acid downregulates TRPV1 receptor via NF-κB and attenuates neuropathic pain in rats with diabetes. CNS Neurosci Ther 26(7):762–772. https://doi.org/10.1111/cns.13303
Nazıroğlu M (2017) Activation of TRPM2 and TRPV1 channels in dorsal root ganglion by NADPH oxidase and protein kinase C molecular pathways: a Patch clamp study. J Mol Neurosci 61(3):425–435. https://doi.org/10.1007/s12031-017-0882-4
Santos CX, Tanaka LY, Wosniak J, Laurindo FR (2009) Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11(10):2409–24027. https://doi.org/10.1089/ars.2009.2625
Sakaguchi R, Mori Y (2020) Transient receptor potential (TRP) channels: Biosensors for redox environmental stimuli and cellular status. Free Radic Biol Med 146:36–44. https://doi.org/10.1016/j.freeradbiomed.2019.10.415
Espino J, Bejarano I, Paredes SD, Barriga C, Rodríguez AB, Pariente JA (2011) Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: relation with its antioxidant actions. J Pineal Res 51(2):195–206. https://doi.org/10.1111/j.1600-079X.2011.00876.x
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18(9):685–716. https://doi.org/10.2165/00002512-200118090-00004
Acknowledgements
The authors wish thanks to technician Fatih Şahin (BSN Health, Analyses, Innovation, Consultancy, Organization, Agriculture, and Industry Ltd., Isparta, Turkey) for helping the patch-clamp analyses.
Funding
This research was supported by the Inonu University, BAPSIS (Project number: TCD-2019–624). Coordinator of the project was Dr. Duzova.
Author information
Authors and Affiliations
Contributions
Dr. Duzova, Dr. Gurbuz and Dr. Akatlı reviewed the current literature, and designed the project. Dr. Cig performed animal care and experiments. Dr. Gurbuz and Dr. Cig performed the rat experimental processes. Dr. Naziroglu performed and analyzed patch clamp, Western blot, and spectrofluorometer tests. Dr. Duzova and Dr. Naziroglu conceptualized this perspective piece, reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants performed by any of the authors. This study was carried out with approvals of Inonu University Medical School Experimental Animals Ethics Committee (2017/A-13) and Experimental Animal Research Center of SDU (Permit Numbers: 2019–06-03. Date 25.04.2019). The authors have no ethical conflicts to disclose.
Consent to Participate
Dr. Duzova, Dr. Gurbuz and Dr. Akatlı reviewed the current literature, and designed the project. Dr. Cig performed animal care and experiments. Dr. Gurbuz and Dr. Cig performed the experimental process. Dr. Naziroglu performed and analyzed patch clamp, Western blot, and spectrofluorometer tests. Dr. Duzova, Dr. Naziroglu, and Dr. Gurbuz conceptualized this perspective piece, reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
Consent for Publication
Last version of the manuscript was approved by the authors before the submission.
Conflict of Interest
The authors have no conflicts of interest to declare.
Research involving Human Participants and/or Animals
Human and human samples were not used in the present study. Rat and rat tissue samples were used in the current study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Düzova, H., Nazıroğlu, M., Çiğ, B. et al. Noopept Attenuates Diabetes-Mediated Neuropathic Pain and Oxidative Hippocampal Neurotoxicity via Inhibition of TRPV1 Channel in Rats. Mol Neurobiol 58, 5031–5051 (2021). https://doi.org/10.1007/s12035-021-02478-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02478-8