Abstract
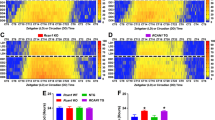
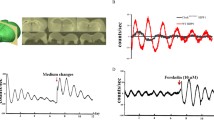
The circadian clock is an endogenous system designed to anticipate and adapt to daily changes in the environment. Alzheimer’s disease (AD) is a progressive neurodegenerative disease, which is more prevalent in patients with type 2 diabetes mellitus (T2DM). However, the effects of circadian disruption on mental and physical health for T2DM patients are not yet fully understood, even though circadian disruption has been confirmed to promote the progression of AD in population. By housing db/db mice on a disrupted (a 6:18 light/dark cycle) circadian rhythm, we assessed the circadian gene expression, body weight, cognitive ability, and AD-related pathophysiology. Our results indicated that housing in these conditions led to disrupted diurnal circadian rhythms in the hippocampus of db/db mice and contributed to their weight gain. In the brain, the circadian-disrupted db/db mice showed a decreased cognitive ability and an increased hyperphosphorylation of tau protein, even though no difference was found in amyloid protein (Aβ) plaque deposition. We also found that the hyperphosphorylated tau protein exhibited more disruptive daily oscillations in db/db mice hippocampus under the 6:18 light/dark cycle. Circadian alterations could promote the development of AD in T2DM.






Similar content being viewed by others
Data Availability
Data will be made available on reasonable quest.
References
Roenneberg T, Merrow M (2005) Circadian clocks-the fall and rise of physiology. Nat Rev Mol Cell Biol 6(12):965–971. https://doi.org/10.1038/nrm1766
Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM (1999) A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell 96(1):57–68. https://doi.org/10.1016/s0092-8674(00)80959-9
Woodie LN, Johnson RM, Ahmed B, Fowler S, Haynes W, Carmona B, Reed M, Suppiramaniam V et al (2020) Western diet-induced obesity disrupts the diurnal rhythmicity of hippocampal core clock gene expression in a mouse model. Brain Behav Immun 88:815–825. https://doi.org/10.1016/j.bbi.2020.05.053
Boles A, Kandimalla R, Reddy PH (2017) Dynamics of diabetes and obesity: epidemiological perspective. Biochim Biophys Acta Mol basis Dis 1863(5):1026–1036. https://doi.org/10.1016/j.bbadis.2017.01.016
WHO (2014) Global status report on noncommunicable diseases 2014.
Mason IC, Qian J, Adler GK, Scheer F (2020) Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 63(3):462–472. https://doi.org/10.1007/s00125-019-05059-6
Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A (2019) Circadian clocks and insulin resistance. Nat Rev Endocrinol 15(2):75–89. https://doi.org/10.1038/s41574-018-0122-1
Buckley TM, Schatzberg AF (2005) On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 90(5):3106–3114. https://doi.org/10.1210/jc.2004-1056
van Raalte DH, Diamant M (2014) Steroid diabetes: from mechanism to treatment? Neth J Med 72(2):62–72
Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S et al (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308(5724):1043–1045. https://doi.org/10.1126/science.1108750
Lee J, Kim MS, Li R, Liu VY, Fu L, Moore DD, Ma K, Yechoor VK (2011) Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets 3(6):381–388. https://doi.org/10.4161/isl.3.6.18157
Rakshit K, Hsu TW, Matveyenko AV (2016) Bmal1 is required for beta cell compensatory expansion, survival and metabolic adaptation to diet-induced obesity in mice. Diabetologia 59(4):734–743. https://doi.org/10.1007/s00125-015-3859-2
Kandimalla R, Thirumala V, Reddy PH (2017) Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim Biophys Acta Mol basis Dis 1863(5):1078–1089. https://doi.org/10.1016/j.bbadis.2016.08.018
Joe E, Ringman JM (2019) Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. Bmj 367:l6217. https://doi.org/10.1136/bmj.l6217
Musiek ES, Xiong DD, Holtzman DM (2015) Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med 47:e148. https://doi.org/10.1038/emm.2014.121
Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS (2011) Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 108(4):1657–1662. https://doi.org/10.1073/pnas.1018375108
Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, Musiek ES (2018) Regulation of amyloid-β dynamics and pathology by the circadian clock. J Exp Med 215(4):1059–1068. https://doi.org/10.1084/jem.20172347
Stevanovic K, Yunus A, Joly-Amado A, Gordon M, Morgan D, Gulick D, Gamsby J (2017) Disruption of normal circadian clock function in a mouse model of tauopathy. Exp Neurol 294:58–67. https://doi.org/10.1016/j.expneurol.2017.04.015
Musiek ES (2015) Circadian clock disruption in neurodegenerative diseases: cause and effect? Front Pharmacol 6:29. https://doi.org/10.3389/fphar.2015.00029
Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M (2004) Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology 63(10):1902–1907. https://doi.org/10.1212/01.wnl.0000144278.79488.dd
Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S et al (2009) Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326(5955):1005–1007. https://doi.org/10.1126/science.1180962
Planel E, Richter KE, Nolan CE, Finley JE, Liu L, Wen Y, Krishnamurthy P, Herman M et al (2007) Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci 27(12):3090–3097. https://doi.org/10.1523/jneurosci.4854-06.2007
Volmar CH, Salah-Uddin H, Janczura KJ, Halley P, Lambert G, Wodrich A, Manoah S, Patel NH et al (2017) M344 promotes nonamyloidogenic amyloid precursor protein processing while normalizing Alzheimer’s disease genes and improving memory. Proc Natl Acad Sci U S A 114(43):E9135–e9144. https://doi.org/10.1073/pnas.1707544114
Hou TY, Zhou Y, Zhu LS, Wang X, Pang P, Wang DQ, Liuyang ZY, Man H et al (2020) Correcting abnormalities in miR-124/PTPN1 signaling rescues tau pathology in Alzheimer’s disease. J Neurochem 154(4):441–457. https://doi.org/10.1111/jnc.14961
Chen JL, Zhang DL, Sun Y, Zhao YX, Zhao KX, Pu D, Xiao Q (2017) Angiotensin-(1-7) administration attenuates Alzheimer’s disease-like neuropathology in rats with streptozotocin-induced diabetes via Mas receptor activation. Neuroscience 346:267–277. https://doi.org/10.1016/j.neuroscience.2017.01.027
Burke SJ, Batdorf HM, Burk DH, Noland RC, Eder AE, Boulos MS, Karlstad MD (2017) Collier JJ (2017) db/db mice exhibit features of human type 2 diabetes that are not present in weight-matched C57BL/6J mice fed a Western diet. J Diabetes Res 8503754:1–17. https://doi.org/10.1155/2017/8503754
Klueh U, Liu Z, Cho B, Ouyang T, Feldman B, Henning TP, Kaur M, Kreutzer D (2006) Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther 8(3):402–412. https://doi.org/10.1089/dia.2006.8.402
Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N et al (2007) Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 49(5):1104–1113. https://doi.org/10.1161/hypertensionaha.106.083568
Karakoc Y, Buruk MS, Aktan B, Kirvar R, Erdogan S, Sahbaz MA, Aksoy S, Gulyasar T (2011) Effects of chronic light/dark cycle on iron zinc and copper levels in different brain regions of rats. Biol Trace Elem Res 144(1-3):1003–1007. https://doi.org/10.1007/s12011-011-9081-2
Tahsili-Fahadan P, Yahyavi-Firouz-Abadi N, Ghahremani MH, Dehpour AR (2005) Effect of light/dark cycle alteration on morphine-induced conditioned place preference. Neuroreport 16(18):2051–2056. https://doi.org/10.1097/00001756-200512190-00017
Li Y, Ma J, Yao K, Su W, Tan B, Wu X, Huang X, Li T et al (2020) Circadian rhythms and obesity: timekeeping governs lipid metabolism. J Pineal Res 69(3):e12682. https://doi.org/10.1111/jpi.12682
Niedhammer I, Lert F, Marne MJ (1996) Prevalence of overweight and weight gain in relation to night work in a nurses’ cohort. Int J Obes Relat Metab Disord 20(7):625–633
Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M et al (2019) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363(6429):880–884. https://doi.org/10.1126/science.aav2546
Zhao HY, Wu HJ, He JL, Zhuang JH, Liu ZY, Huang LQ, Zhao ZX (2017) Chronic sleep restriction induces cognitive deficits and cortical beta-amyloid deposition in mice via BACE1-antisense activation. CNS Neurosci Ther 23(3):233–240. https://doi.org/10.1111/cns.12667
Cho K (2001) Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci 4(6):567–568. https://doi.org/10.1038/88384
Braak H, Del Tredici K (2011) The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121(2):171–181. https://doi.org/10.1007/s00401-010-0789-4
Guha S, Johnson GVW, Nehrke K (2020) The crosstalk between pathological tau phosphorylation and mitochondrial dysfunction as a key to understanding and treating Alzheimer’s disease. Mol Neurobiol 57(12):5103–5120. https://doi.org/10.1007/s12035-020-02084-0
Qiu H, Zhong R, Liu H, Zhang F, Li S, Le W (2016) Chronic sleep deprivation exacerbates learning-memory disability and Alzheimer’s disease-like pathologies in AβPP(swe)/PS1(ΔE9) mice. J Alzheim Disease 50(3):669–685. https://doi.org/10.3233/jad-150774
Belfiore R, Rodin A, Ferreira E, Velazquez R, Branca C, Caccamo A, Oddo S (2019) Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 18(1):e12873. https://doi.org/10.1111/acel.12873
Pu D, Zhao Y, Chen J, Sun Y, Lv A, Zhu S, Luo C, Zhao K et al (2018) Protective effects of sulforaphane on cognitive impairments and AD-like lesions in diabetic mice are associated with the upregulation of Nrf2 transcription activity. Neuroscience 381:35–45. https://doi.org/10.1016/j.neuroscience.2018.04.017
Wu Y, Yuan Y, Wu C, Jiang T, Wang B, Xiong J, Zheng P, Li Y et al (2020) The reciprocal causation of the ASK1-JNK1/2 pathway and endoplasmic reticulum stress in diabetes-induced cognitive decline. Front Cell Dev Biol 8:602. https://doi.org/10.3389/fcell.2020.00602
Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F, Chang H, Zhao G et al (2020) Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice. Clin Sci (London, England : 1979) 134(16):2161–2175. https://doi.org/10.1042/cs20200530
Kalani A, Chaturvedi P, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N (2017) Dementia-like pathology in type-2 diabetes: a novel microRNA mechanism. Mol Cell Neurosci 80:58–65. https://doi.org/10.1016/j.mcn.2017.02.005
Acknowledgements
We thank Danpei Li and Li Huang from Huazhong University of Science and Technology for the technical advice and assistance with this study.
Funding
This work was supported by the National Natural Science Foundation of China (Grants 81670754, 81800686, and 81974114) and funds of Jie Chu Jing Ying Foundation (Grants 2018076).
Author information
Authors and Affiliations
Contributions
YY and JH conceived and designed the study and wrote the manuscript. YY, XS, KD, and XY secured the study’s funding. JH, XP, RF, KD, XS, SZ, and XY acquired and analyzed the data. All authors revised the article and approved its final version.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PNG 28 kb)
Rights and permissions
About this article
Cite this article
Huang, J., Peng, X., Fan, R. et al. Disruption of Circadian Clocks Promotes Progression of Alzheimer’s Disease in Diabetic Mice. Mol Neurobiol 58, 4404–4412 (2021). https://doi.org/10.1007/s12035-021-02425-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02425-7