Abstract
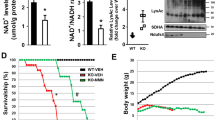
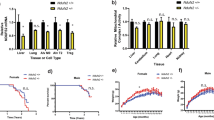
Mitochondrial diseases (MD), such as Leigh syndrome (LS), present with severe neurological and muscular phenotypes in patients, but have no known cure and limited treatment options. Based on their neuroprotective effects against other neurodegenerative diseases in vivo and their positive impact as an antioxidant against complex I deficiency in vitro, we investigated the potential protective effect of metallothioneins (MTs) in an Ndufs4 knockout mouse model (with a very similar phenotype to LS) crossed with an Mt1 overexpressing mouse model (TgMt1). Despite subtle reductions in the expression of neuroinflammatory markers GFAP and IBA1 in the vestibular nucleus and hippocampus, we found no improvement in survival, growth, locomotor activity, balance, or motor coordination in the Mt1 overexpressing Ndufs4−/− mice. Furthermore, at a cellular level, no differences were detected in the metabolomics profile or gene expression of selected one-carbon metabolism and oxidative stress genes, performed in the brain and quadriceps, nor in the ROS levels of macrophages derived from these mice. Considering these outcomes, we conclude that MT1, in general, does not protect against the impaired motor activity or improve survival in these complex I–deficient mice. The unexpected absence of increased oxidative stress and metabolic redox imbalance in this MD model may explain these observations. However, tissue-specific observations such as the mildly reduced inflammation in the hippocampus and vestibular nucleus, as well as differential MT1 expression in these tissues, may yet reveal a tissue- or cell-specific role for MTs in these mice.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, FHvdW, upon reasonable request.
Abbreviations
- 1C:
-
One-carbon
- ADP:
-
Adenosine diphosphate
- BMDM:
-
Bone marrow–derived macrophage
- BH-FDR:
-
Benjamini–Hochberg adjustment to control the rate of false discovery
- CI:
-
Complex I
- CII:
-
Complex II
- CIII:
-
Complex III
- CIV:
-
Complex IV
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DNA:
-
Deoxyribonucleic acid
- dNTP:
-
Deoxynucleotide triphosphate
- GC-TOF-MS:
-
Gas chromatography time-of-flight mass spectrometry
- GFAP:
-
Glial fibrillary acidic protein
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidised glutathione
- H2DCF-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- H2O2 :
-
Hydrogen peroxide
- IBA1:
-
Ionised calcium binding adapter molecule 1
- IL6:
-
Interleukin 6
- IL12:
-
Interleukin 12
- KO:
-
Ndufs4 knockout
- KO OVER:
-
Ndufs4 knockout Mt1 overexpressing
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- LS:
-
Leigh syndrome
- MCP1:
-
Monocyte chemoattractant protein 1
- MCSF:
-
Macrophage colony-stimulating factor
- mRNA:
-
Messenger RNA
- MD:
-
Mitochondrial disease
- MT:
-
Metallothionein
- NAD+ :
-
Oxidised nicotinamide adenine dinucleotide
- NADH:
-
Reduced nicotinamide adenine dinucleotide
- NDUFS4:
-
NADH:ubiquinone oxidoreductase subunit S4
- NMR:
-
Nuclear magnetic resonance spectroscopy
- NWU:
-
North-West University
- OVER:
-
Mt1 overexpressing
- P:
-
Postnatal day
- PARP:
-
Poly[adenine diphosphate (ADP)-ribose] polymerase
- PBS:
-
Physiological buffered saline
- PCR:
-
Polymerase chain reaction
- RC:
-
Respiratory chain
- RNA:
-
Ribonucleic acid
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SI:
-
Supporting information
- TNF:
-
Tumour necrosis factor
- UCS:
-
Unit citrate synthase
- WT:
-
Wild type
References
Distelmaier F, Koopman WJ, van den Heuvel LP, Rodenburg RJ, Mayatepek E, Willems PH, Smeitink JA (2009) Mitochondrial complex I deficiency: from organelle dysfunction to clinical disease. Brain 132(Pt 4):833–842. https://doi.org/10.1093/brain/awp058
Reinecke F, Smeitink JA, van der Westhuizen FH (2009) OXPHOS gene expression and control in mitochondrial disorders. Biochim Biophys Acta 1792(12):1113–1121. https://doi.org/10.1016/j.bbadis.2009.04.003
Rodenburg RJ (2016) Mitochondrial complex I-linked disease. Biochim Biophys Acta 1857(7):938–945. https://doi.org/10.1016/j.bbabio.2016.02.012
Valsecchi F, Koopman WJ, Manjeri GR, Rodenburg RJ, Smeitink JA, Willems PH (2010) Complex I disorders: causes, mechanisms, and development of treatment strategies at the cellular level. Dev Disabil Res Rev 16(2):175–182. https://doi.org/10.1002/ddrr.107
de Haas R, Das D, Garanto A, Renkema HG, Greupink R, van den Broek P, Pertijs J, Collin RWJ et al (2017) Therapeutic effects of the mitochondrial ROS-redox modulator KH176 in a mammalian model of Leigh disease. Sci Rep 7(1):11733. https://doi.org/10.1038/s41598-017-09417-5
Koene S, Willems PH, Roestenberg P, Koopman WJ, Smeitink JA (2011) Mouse models for nuclear DNA-encoded mitochondrial complex I deficiency. J Inherit Metab Dis 34(2):293–307. https://doi.org/10.1007/s10545-009-9005-x
Koopman WJ, Verkaart S, van Emst-de Vries SE, Grefte S, Smeitink JA, Nijtmans LG, Willems PH (2008) Mitigation of NADH: ubiquinone oxidoreductase deficiency by chronic Trolox treatment. Biochim Biophys Acta 1777(7–8):853–859. https://doi.org/10.1016/j.bbabio.2008.03.028
Maret W (2000) The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr 130(5):1455S–1458S
Palmiter RD (1998) The elusive function of metallothioneins. Proc Natl Acad Sci U S A 95(15):8428–8430. https://doi.org/10.1073/pnas.95.15.8428
Babula P, Masarik M, Adam V, Eckschlager T, Stiborova M, Trnkova L, Skutkova H, Provaznik I et al (2012) Mammalian metallothioneins: properties and functions. Metallomics 4(8):739–750. https://doi.org/10.1039/c2mt20081c
Dalton TP, Li Q, Bittel D, Liang L, Andrews GK (1996) Oxidative stress activates metal-responsive transcription factor-1 binding activity. Occupancy in vivo of metal response elements in the metallothionein-I gene promoter. J Biol Chem 271(42):26233–26241. https://doi.org/10.1074/jbc.271.42.26233
Juarez-Rebollar D, Rios C, Nava-Ruiz C, Mendez-Armenta M (2017) Metallothionein in brain disorders. Oxidative Med Cell Longev 2017:5828056–5828012. https://doi.org/10.1155/2017/5828056
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V et al (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14(3):6044–6066. https://doi.org/10.3390/ijms14036044
West AK, Hidalgo J, Eddins D, Levin ED, Aschner M (2008) Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology 29(3):489–503. https://doi.org/10.1016/j.neuro.2007.12.006
Artells E, Palacios Ò, Capdevila M, Atrian S (2013) Mammalian MT1 and MT2 metallothioneins differ in their metal binding abilities. Metallomics 5(10):1397–1410
Comes G, Fernandez-Gayol O, Molinero A, Giralt M, Capdevila M, Atrian S, Hidalgo J (2019) Mouse metallothionein-1 and metallothionein-2 are not biologically interchangeable in an animal model of multiple sclerosis, EAE. Metallomics 11(2):327–337
Miles A, Hawksworth G, Beattie J, Rodilla V (2000) Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol 35(1):35–70
Lindeque JZ, Levanets O, Louw R, van der Westhuizen FH (2010) The involvement of metallothioneins in mitochondrial function and disease. Curr Protein Pept Sci 11(4):292–309
Reinecke F, Levanets O, Olivier Y, Louw R, Semete B, Grobler A, Hidalgo J, Smeitink J et al (2006) Metallothionein isoform 2A expression is inducible and protects against ROS-mediated cell death in rotenone-treated HeLa cells. Biochem J 395:405–415. https://doi.org/10.1042/Bj20051253
Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K, Herrmann JM (2005) A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 121(7):1059–1069
Ye B, Maret W, Vallee BL (2001) Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci 98(5):2317–2322
Zhou Z, Kang YJ (2000) Immunocytochemical localization of metallothionein and its relation to doxorubicin toxicity in transgenic mouse heart. Am J Pathol 156(5):1653–1662
Ferreira ADC, Ciriolo MR, Marcocci L, Rotilio G (1993) Copper (I) transfer into metallothionein mediated by glutathione. Biochem J 292(3):673–676
Maret W (1994) Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci 91(1):237–241
Rofe AM, Philcox JC, Coyle P (1996) Trace metal, acute phase and metabolic response to endotoxin in metallothionein-null mice. Biochem J 314(Pt 3):793–797. https://doi.org/10.1042/bj3140793
Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ (2006) Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 48(8):1688–1697. https://doi.org/10.1016/j.jacc.2006.07.022
Ding H, Zhou B, Liu L, Cheng S (2002) Oxidative stress and metallothionein expression in the liver of rats with severe thermal injury. Burns 28(3):215–221
Quesada AR, Byrnes RW, Krezoski SO, Petering DH (1996) Direct reaction of H2O2with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites. Arch Biochem Biophys 334(2):241–250
Wu H-Y, Kang YJ (1998) Inhibition of buthionine sulfoximine-enhanced doxorubicin toxicity in metallothionein overexpressing transgenic mouse heart. J Pharmacol Exp Ther 287(2):515–520
Merten KE, Feng W, Zhang L, Pierce W, Cai J, Klein JB, Kang YJ (2005) Modulation of cytochrome C oxidase-va is possibly involved in metallothionein protection from doxorubicin cardiotoxicity. J Pharmacol Exp Ther 315(3):1314–1319
Carrasco J, Penkowa M, Hadberg H, Molinero A, Hidalgo J (2000) Enhanced seizures and hippocampal neurodegeneration following kainic acid-induced seizures in metallothionein-I + II-deficient mice. Eur J Neurosci 12(7):2311–2322. https://doi.org/10.1046/j.1460-9568.2000.00128.x
Ebadi M, Brown-Borg H, El Refaey H, Singh BB, Garrett S, Shavali S, Sharma SK (2005) Metallothionein-mediated neuroprotection in genetically engineered mouse models of Parkinson’s disease. Brain Res Mol Brain Res 134(1):67–75. https://doi.org/10.1016/j.molbrainres.2004.09.011
Eidizadeh A, Khajehalichalehshtari M, Freyer D, Trendelenburg G (2015) Assessment of the therapeutic potential of metallothionein-II application in focal cerebral ischemia in vitro and in vivo. PLoS One 10(12):e0144035. https://doi.org/10.1371/journal.pone.0144035
Giralt M, Penkowa M, Lago N, Molinero A, Hidalgo J (2002) Metallothionein-1+2 protect the CNS after a focal brain injury. Exp Neurol 173(1):114–128. https://doi.org/10.1006/exnr.2001.7772
Hidalgo J, Penkowa M, Espejo C, Martinez-Caceres EM, Carrasco J, Quintana A, Molinero A, Florit S et al (2006) Expression of metallothionein-I, -II, and -III in Alzheimer disease and animal models of neuroinflammation. Exp Biol Med (Maywood) 231(9):1450–1458. https://doi.org/10.1177/153537020623100902
Kim JH, Nam YP, Jeon SM, Han HS, Suk K (2012) Amyloid neurotoxicity is attenuated by metallothionein: dual mechanisms at work. J Neurochem 121(5):751–762
Miyazaki I, Asanuma M, Hozumi H, Miyoshi K, Sogawa N (2007) Protective effects of metallothionein against dopamine quinone-induced dopaminergic neurotoxicity. FEBS Lett 581(25):5003–5008. https://doi.org/10.1016/j.febslet.2007.09.046
Santos CR, Martinho A, Quintela T, Goncalves I (2012) Neuroprotective and neuroregenerative properties of metallothioneins. IUBMB Life 64(2):126–135. https://doi.org/10.1002/iub.585
Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP (2003) Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem 278(22):19603–19610. https://doi.org/10.1074/jbc.M209359200
Quesada AR, Byrnes RW, Krezoski SO, Petering DH (1996) Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites. Arch Biochem Biophys 334(2):241–250. https://doi.org/10.1006/abbi.1996.0452
Van Der Westhuizen F, Van Den Heuvel L, Smeets R, Veltman J, Pfundt R, Van Kessel A, Ursing B, Smeitink J (2003) Human mitochondrial complex I deficiency: investigating transcriptional responses by microarray. Neuropediatrics 34(01):14–22
Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD (2008) Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab 7(4):312–320. https://doi.org/10.1016/j.cmet.2008.02.004
Palmiter RD, Sandgren EP, Koeller DM, Brinster RL (1993) Distal regulatory elements from the mouse metallothionein locus stimulate gene-expression in transgenic mice. Mol Cell Biol 13(9):5266–5275
Nicholson JK, Lindon JC (2008) Systems biology: metabonomics. Nature 455(7216):1054–1056
Valsecchi F, Monge C, Forkink M, de Groof AJ, Benard G, Rossignol R, Swarts HG, van Emst-de Vries SE et al (2012) Metabolic consequences of NDUFS4 gene deletion in immortalized mouse embryonic fibroblasts. Biochim Biophys Acta 1817(10):1925–1936. https://doi.org/10.1016/j.bbabio.2012.03.006
Rodenburg RJ (2011) Biochemical diagnosis of mitochondrial disorders. J Inherit Metab Dis 34(2):283–292. https://doi.org/10.1007/s10545-010-9081-y
Robinson J, Brent L, Sumegi B, Srere P (1987) An enzymatic approach to the study of the Krebs tricarboxylic acid cycle. Mitochondria: a practical approach:153–170
Shepherd D, Garland P (1969) The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J 114(3):597–610
Amend SR, Valkenburg KC, Pienta KJ (2016) Murine hind limb long bone dissection and bone marrow isolation. J Vis Exp 110. https://doi.org/10.3791/53936
Manzanero S (2012) Generation of mouse bone marrow-derived macrophages. Methods Mol Biol 844:177–181. https://doi.org/10.1007/978-1-61779-527-5_12
Marim FM, Silveira TN, Lima DS Jr, Zamboni DS (2010) A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One 5(12):e15263. https://doi.org/10.1371/journal.pone.0015263
Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. Curr Protoc Immunol Chapter 14(Unit 14):11. https://doi.org/10.1002/0471142735.im1401s83
Carlson CG, Rutter J, Bledsoe C, Singh R, Hoff H, Bruemmer K, Sesti J, Gatti F et al (2010) A simple protocol for assessing inter-trial and inter-examiner reliability for two noninvasive measures of limb muscle strength. J Neurosci Methods 186(2):226–230. https://doi.org/10.1016/j.jneumeth.2009.11.006
Luong TN, Carlisle HJ, Southwell A, Patterson PH (2011) Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp 49. https://doi.org/10.3791/2376
Deacon RM (2013) Measuring motor coordination in mice. J Vis Exp 75:e2609. https://doi.org/10.3791/2609
Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD (2010) Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci USA 107(24):10996–11001. https://doi.org/10.1073/pnas.1006214107
Corasaniti M, Maiuolo J, Maida S, Fratto V, Navarra M, Russo R, Amantea D, Morrone L et al (2007) Cell signaling pathways in the mechanisms of neuroprotection afforded by bergamot essential oil against NMDA-induced cell death in vitro. Br J Pharmacol 151(4):518–529
Janda E, Lascala A, Carresi C, Parafati M, Aprigliano S, Russo V, Savoia C, Ziviani E et al (2015) Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: implications for neuroprotection. Autophagy 11(7):1063–1080
Lewies A, Wentzel JF, Miller HC, Du Plessis LH (2018) The antimicrobial peptide nisin Z induces selective toxicity and apoptotic cell death in cultured melanoma cells. Biochimie 144:28–40
Terburgh K, Lindeque Z, Mason S, Van der Westhuizen F, Louw R (2019) Metabolomics of Ndufs4−/− skeletal muscle: adaptive mechanisms converge at the ubiquinone-cycle. Biochim Biophys Acta (BBA) Mol Basis Dis 1865(1):98–106
Mason S, Terburgh K, Louw R (2018) Miniaturized (1)H-NMR method for analyzing limited-quantity samples applied to a mouse model of Leigh disease. Metabolomics 14(6):74. https://doi.org/10.1007/s11306-018-1372-6
Lindeque JZ, Hidalgo J, Louw R, van der Westhuizen FH (2013) Systemic and organ specific metabolic variation in metallothionein knockout mice challenged with swimming exercise. Metabolomics 9(2):418–432
Mels CM, Jansen van Rensburg P, van der Westhuizen FH, Pretorius PJ, Erasmus E (2011) Increased excretion of c4-carnitine species after a therapeutic acetylsalicylic acid dose: evidence for an inhibitory effect on short-chain fatty acid metabolism. ISRN Pharmacol 2011:851870–851878. https://doi.org/10.5402/2011/851870
Gullberg J, Jonsson P, Nordstrom A, Sjostrom M, Moritz T (2004) Design of experiments: an efficient strategy to identify factors influencing extraction and derivatization of Arabidopsis thaliana samples in metabolomic studies with gas chromatography/mass spectrometry. Anal Biochem 331(2):283–295. https://doi.org/10.1016/j.ab.2004.04.037
Pfaffl MW (2007) Relative quantification. In: Real-time PCR. Taylor & Francis, pp 89–108
Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46(W1):W486–W494. https://doi.org/10.1093/nar/gky310
Iszard MB, Liu J, Liu Y, Dalton T, Andrews GK, Palmiter RD, Klaassen CD (1995) Characterization of metallothionein-I-transgenic mice. Toxicol Appl Pharmacol 133(2):305–312. https://doi.org/10.1006/taap.1995.1155
Ono S, Koropatnick DJ, Cherian MG (1997) Regional brain distribution of metallothionein, zinc and copper in toxic milk mutant and transgenic mice. Toxicology 124(1):1–10. https://doi.org/10.1016/s0300-483x(97)00133-9
Manso Y, Comes G, López-Ramos JC, Belfiore M, Molinero A, Giralt M, Carrasco J, Adlard PA et al (2016) Overexpression of metallothionein-1 modulates the phenotype of the Tg2576 mouse model of Alzheimer’s disease. J Alzheimers Dis 51(1):81–95
Penkowa M, Florit S, Giralt M, Quintana A, Molinero A, Carrasco J, Hidalgo J (2005) Metallothionein reduces central nervous system inflammation, neurodegeneration, and cell death following kainic acid-induced epileptic seizures. J Neurosci Res 79(4):522–534. https://doi.org/10.1002/jnr.20387
Calvaruso MA, Willems P, van den Brand M, Valsecchi F, Kruse S, Palmiter R, Smeitink J, Nijtmans L (2012) Mitochondrial complex III stabilizes complex I in the absence of NDUFS4 to provide partial activity. Hum Mol Genet 21(1):115–120. https://doi.org/10.1093/hmg/ddr446
Alam MT, Manjeri GR, Rodenburg RJ, Smeitink JA, Notebaart RA, Huynen M, Willems PH, Koopman WJ (2015) Skeletal muscle mitochondria of NDUFS4-/- mice display normal maximal pyruvate oxidation and ATP production. Biochim Biophys Acta 1847(6–7):526–533. https://doi.org/10.1016/j.bbabio.2015.02.006
Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J et al (2016) Hypoxia as a therapy for mitochondrial disease. Science 352(6281):54–61. https://doi.org/10.1126/science.aad9642
de Haas R, Russel FG, Smeitink JA (2016) Gait analysis in a mouse model resembling Leigh disease. Behav Brain Res 296:191–198. https://doi.org/10.1016/j.bbr.2015.09.006
Felici R, Cavone L, Lapucci A, Guasti D, Bani D, Chiarugi A (2014) PARP inhibition delays progression of mitochondrial encephalopathy in mice. Neurotherapeutics 11(3):651–664. https://doi.org/10.1007/s13311-014-0285-y
Hidalgo J, Aschner M, Zatta P, Vasak M (2001) Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull 55(2):133–145
Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J et al (2013) mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342(6165):1524–1528. https://doi.org/10.1126/science.1244360
Quintana A, Zanella S, Koch H, Kruse SE, Lee D, Ramirez JM, Palmiter RD (2012) Fatal breathing dysfunction in a mouse model of Leigh syndrome. J Clin Invest 122(7):2359–2368. https://doi.org/10.1172/Jci62923
Schauwecker PE (2011) The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res 97(1–2):1–11. https://doi.org/10.1016/j.eplepsyres.2011.09.005
Loos M, Koopmans B, Aarts E, Maroteaux G, van der Sluis S, Neuro BMPC, Verhage M, Smit AB (2015) Within-strain variation in behavior differs consistently between common inbred strains of mice. Mamm Genome 26(7–8):348–354. https://doi.org/10.1007/s00335-015-9578-7
Mocchegiani E, Giacconi R, Fattoretti P, Casoli T, Cipriano C, Muti E, Malavolta M, DiStefano G et al (2004) Metallothionein isoforms (I+ II and III) and interleukin-6 in the hippocampus of old rats: may their concomitant increments lead to neurodegeneration? Brain Res Bull 63(2):133–142
Choudhuri S, Kramer KK, Berman NE, Dalton TP, Andrews GK, Klaassen CD (1995) Constitutive expression of metallothionein genes in mouse brain. Toxicol Appl Pharmacol 131(1):144–154. https://doi.org/10.1006/taap.1995.1056
Carrasco J, Giralt M, Molinero A, Penkowa M, Moos T, Hidalgo J (1999) Metallothionein (MT)-III: generation of polyclonal antibodies, comparison with MT-I+II in the freeze lesioned rat brain and in a bioassay with astrocytes, and analysis of Alzheimer’s disease brains. J Neurotrauma 16(11):1115–1129. https://doi.org/10.1089/neu.1999.16.1115
Carrasco J, Giralt M, Penkowa M, Stalder AK, Campbell IL, Hidalgo J (2000) Metallothioneins are upregulated in symptomatic mice with astrocyte-targeted expression of tumor necrosis factor-alpha. Exp Neurol 163(1):46–54. https://doi.org/10.1006/exnr.1999.7335
Penkowa M, Quintana A, Carrasco J, Giralt M, Molinero A, Hidalgo J (2004) Metallothionein prevents neurodegeneration and central nervous system cell death after treatment with gliotoxin 6-aminonicotinamide. J Neurosci Res 77(1):35–53. https://doi.org/10.1002/jnr.20154
Piroli GG, Manuel AM, Clapper AC, Walla MD, Baatz JE, Palmiter RD, Quintana A, Frizzell N (2016) Succination is increased on select proteins in the brainstem of the NADH dehydrogenase (ubiquinone) Fe-S protein 4 (Ndufs4) knockout mouse, a model of leigh syndrome. Mol Cell Proteomics 15(2):445–461
Bao XR, Ong SE, Goldberger O, Peng J, Sharma R, Thompson DA, Vafai SB, Cox AG et al (2016) Mitochondrial dysfunction remodels one-carbon metabolism in human cells. Elife 5. https://doi.org/10.7554/eLife.10575
Nikkanen J, Forsstrom S, Euro L, Paetau I, Kohnz RA, Wang L, Chilov D, Viinamaki J et al (2016) Mitochondrial DNA replication defects disturb cellular dNTP pools and remodel one-carbon metabolism. Cell Metab 23(4):635–648. https://doi.org/10.1016/j.cmet.2016.01.019
Jin Z, Wei W, Yang M, Du Y, Wan Y (2014) Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab 20(3):483–498. https://doi.org/10.1016/j.cmet.2014.07.011
Valsecchi F, Grefte S, Roestenberg P, Joosten-Wagenaars J, Smeitink JA, Willems PH, Koopman WJ (2013) Primary fibroblasts of NDUFS4(-/-) mice display increased ROS levels and aberrant mitochondrial morphology. Mitochondrion 13(5):436–443. https://doi.org/10.1016/j.mito.2012.12.001
Subramanian Vignesh K, Deepe G Jr (2017) Metallothioneins: emerging modulators in immunity and infection. Int J Mol Sci 18(10):2197
Barbato JC, Catanescu O, Murray K, DiBello PM, Jacobsen DW (2007) Targeting of metallothionein by L-homocysteine: a novel mechanism for disruption of zinc and redox homeostasis. Arterioscler Thromb Vasc Biol 27(1):49–54. https://doi.org/10.1161/01.ATV.0000251536.49581.8a
Esterhuizen K, van der Westhuizen FH, Louw R (2017) Metabolomics of mitochondrial disease. Mitochondrion 35:97–110. https://doi.org/10.1016/j.mito.2017.05.012
Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C et al (2015) Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metab 21(6):845–854. https://doi.org/10.1016/j.cmet.2015.04.016
Hidalgo J, Garcia A, Oliva AM, Giralt M, Gasull T, Gonzalez B, Milnerowicz H, Wood A et al (1994) Effect of zinc, copper and glucocorticoids on metallothionein levels of cultured neurons and astrocytes from rat brain. Chem Biol Interact 93(3):197–219
Zimin PI, Woods CB, Quintana A, Ramirez JM, Morgan PG, Sedensky MM (2016) Glutamatergic neurotransmission links sensitivity to volatile anesthetics with mitochondrial function. Curr Biol 26(16):2194–2201. https://doi.org/10.1016/j.cub.2016.06.020
Bolea I, Gella A, Sanz E, Prada-Dacasa P, Menardy F, Bard AM, Machuca-Márquez P, Eraso-Pichot A et al (2019) Defined neuronal populations drive fatal phenotype in a mouse model of Leigh syndrome. Elife 8
Kayser EB, Sedensky MM, Morgan PG (2016) Region-specific defects of respiratory capacities in the Ndufs4(KO) mouse brain. PLoS One 11(1):e0148219. https://doi.org/10.1371/journal.pone.0148219
Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J et al (2015) Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160(1–2):177–190
Jain IH, Zazzeron L, Goldberger O, Marutani E, Wojtkiewicz GR, Ast T, Wang H, Schleifer G et al (2019) Leigh syndrome mouse model can be rescued by interventions that normalize brain hyperoxia, but not HIF activation. Cell Metab 30(4):824–832 e823
Lee CF, Caudal A, Abell L, Gowda GN, Tian R (2019) Targeting NAD+ metabolism as interventions for mitochondrial disease. Sci Rep 9(1):1–10
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40(2):310–322
Wang S-H, Li L-H, Zou D-M, Zheng X-M, Deng J (2020) Roles of the mammalian target of rapamycin (mTOR) signaling pathway in the repair of hyperoxia-induced acute lung injury. Adv Clin Exp Med 29(1):13–23
Acknowledgements
We thank Kobus Venter and Antoinette Fick from the Pre-Clinical Drug Development Platform (PCDDP, NWU, RSA) for their assistance regarding animal handling. We also thank Dr. De Wet Wolmarans from the Department of Pharmacology (NWU, Potchefstroom, South Africa) for the use of his open field testing equipment and the Instrument Making Department of the NWU for the construction of all other phenotyping equipment. We lastly thank Prof. Juan Hidalgo at the Institut de Neurosciencies at the Autonomous University of Barcelona for the generous provision of the MT antibodies used in the immunohistochemistry analyses.
Funding
This work was supported by the National Research Foundation of South Africa (NRF; Grant Number 92736). AQ is a Ramón y Cajal fellow (RyC-2012-11873) and received funds from the European Research Council (starting grant NEUROMITO, ERC-2014-StG-638106), MINECO Proyectos I+D de Excelencia (SAF2014-57981P), MINECO Proyectos I+D “Retos Investigación” (SAF2017–88108-R), and AGAUR (2017SGR-323).
Author information
Authors and Affiliations
Contributions
All authors reviewed, edited, and approved the final manuscript version for submission, and all authors were involved with the design of the methodology. Hayley Christy Miller: conceptualisation, formal analysis, investigation, writing—original draft, visualisation. Roan Louw: conceptualisation, writing—original draft. Michelle Mereis: investigation, visualisation. Gerda Venter: conceptualisation. John-Drew Boshoff: formal analysis, investigation, visualisation. Liesel Mienie: formal analysis, investigation, visualisation. Mari van Reenen: conceptualisation, formal analysis. Marianne Venter: formal analysis. Jeremie Zander Lindeque: formal analysis. Adán Domínguez-Martínez: formal analysis, visualisation. Albert Quintana: conceptualisation, investigation, resources, visualisation, Supervision. Francois Hendrikus van der Westhuizen: conceptualisation, writing—original draft, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The AnimCare animal research ethics committee of the NWU approved (approval numbers NWU-00364-16-A5 and NWU-00001-15-A5) the animal protocols used in this study. All animals were maintained, and all procedures performed, in accordance with the code of ethics in research, training, and testing of drugs in South Africa and complied with national legislation.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Disclaimer
Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the NRF.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 764 kb).
Rights and permissions
About this article
Cite this article
Miller, H.C., Louw, R., Mereis, M. et al. Metallothionein 1 Overexpression Does Not Protect Against Mitochondrial Disease Pathology in Ndufs4 Knockout Mice. Mol Neurobiol 58, 243–262 (2021). https://doi.org/10.1007/s12035-020-02121-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02121-y