Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is abundantly expressed in the hypothalamus and contributes to hypothalamic functions, including appetite regulation. Although food intake is suggested to be decreased in PACAP (−/−) mice, the detailed mechanisms are still being discussed. We sought to investigate this link. The food consumption at 8 h after refeeding in the (−/−) mice who had fasted for 2 days was significantly lower than in the PACAP (+/+) mice. The nocturnal and daily food intake of (−/−) mice was significantly lower than those of (+/+) mice, but the diurnal food intake showed a tendency to increase. mRNA expression levels of agouti-related peptide (AgRP) were decreased, but those of proopiomelanocortin (POMC) were increased in the hypothalamus of (−/−) mice 4 h after refeeding. Furthermore, intracerebroventricular administration of a PACAP receptor antagonist, PACAP6–38 (1 nmol/4 μL/mouse), decreased food intake and body weight 1, 2, and 4 h after refeeding, as well as expression levels of AgRP at 4 h after refeeding in (+/+) mice. The selective overexpression of PACAP by the infection of an adeno-associated virus in the ventromedial hypothalamus (VMH) resulted in an increase in food intake and AgRP expression in the nocturnal period in addition to the increased food intake at 8 h after refeeding. These results suggest that food intake behavior in mice is triggered by the increase in PACAP expression in the VMH via modulation of AgRP expression in the hypothalamus, pointing to PACAP inhibition as a potential strategy for the development of anti-obesity drugs.
Similar content being viewed by others
Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP), a member of the vasoactive intestinal polypeptide (VIP)/secretin/glucagon/growth hormone-releasing hormone family of neuropeptides, was originally isolated from ovine hypothalamus by its potent activity in stimulating cyclic adenosine 3′, 5′-monophosphate (cAMP) production in rat anterior pituitary cells [1, 2]. There are three distinct G protein-coupled receptors mediating the actions of PACAP and VIP. The PACAP type I (PAC1) receptor, which is coupled primarily to adenylate cyclase/protein kinase A, binds the two forms of PACAP, the 38 amino acid form (PACAP38) and the C-terminal truncated form (PACAP27), with high affinity. Meanwhile, VPAC1 and VPAC2 receptors, which are also principally coupled to adenylate cyclase, bind both PACAP and VIP with similar affinities [3,4,5].
PACAP is a pleiotropic neuropeptide which is involved in a variety physiological function, including thermogenesis, locomotor activity, mobilization of energy stores, auditory role, and appetite [6,7,8]. Although it has been proposed that PACAP and its receptors are strongly expressed in the hypothalamus, and responsible for appetite regulation [4], reports are discrepant. Several studies have reported that exogenous injections of PACAP suppressed appetite. For example, PACAP injection into the lateral cerebral ventricles [9,10,11], ventromedial hypothalamus (VMH), paraventricular hypothalamus (PVH) [11,12,13], central amygdala (CeA) [14], and the bed nucleus of the stria terminalis (BNST) [15] decreased food intake. In contrast, several reports have demonstrated that daily food intake of PACAP (−/−) mice was decreased in comparison with that of PACAP (+/+) littermates [16, 17]. Thus, studies using (−/−) mice indicated that endogenous PACAP was orexigenic rather than anorexigenic. However, the mechanism by which PACAP mediates its orexigenic effect remains under discussion.
The arcuate nucleus (ARC) is one of the hypothalamic nuclei and includes two major neuronal populations—agouti-related peptide (AgRP)/neuropeptide Y (NPY) and cocaine—and amphetamine-regulated transcript (CART)/proopiomelanocortin (POMC) neurons—which are well recognized as critical regulators of appetite [18,19,20]. Co-expression of AgRP and NPY is found in the ventromedial ARC neurons, and this neuronal group plays a role in promoting food intake in the case of lack of food [21]. For instance, acute ablation of AgRP neurons in mice resulted in the suppression of feeding [22], and fasting increased expression levels of AgRP/NPY [18]. It has also been elucidated that AgRP neurons are inhibited by both insulin and leptin [23, 24], which are known to interact synergistically to suppress food intake and body weight [25]. In contrast, AgRP neurons were activated by ghrelin [26], which has been shown to vigorously prompt food intake in multiple species, including humans [19, 27, 28]. In parallel, in the ventrolateral ARC, there are neurons that co-express CART and POMC, whose activity is known to be reduced in the case of lack of food, but activated in the case of satiation [19]. Notably, PACAP is demonstrated to interact with both of these neuronal subpopulations via PAC1 and VPAC2 receptors expressed on POMC neurons [6] and PAC1 receptors expressed on AgRP neurons [29]. For instance, Mizuno et al. (1998) found that although intracerebroventricular (i.c.v.) administration of PACAP had an anorexigenic effect, PACAP increased the expression of NPY [30]. In addition, the intracellular calcium level was increased in isolated NPY neurons by exposure to PACAP [16]. Likewise, patch-clamp analysis in hypothalamic slices revealed that PACAP increased membrane potential and firing probability of AgRP neurons [31]. Meanwhile, it was also shown that i.c.v. injection of PACAP increased POMC expression as well as cFos expression in POMC neurons [9, 10]. Moreover, intra-VMH injection of PACAP also resulted in an increase in the expression of POMC [12]. On the basis of these observations, we hypothesized that PACAP might regulate appetite by modulating neuropeptide expression in the hypothalamus, and, more importantly, PACAP might potentially affect both orexigenic (AgRP/NPY) and anorexigenic (CART/POMC) systems.
To address these issues in this study, we attempted to reevaluate food intake behavior and expression levels of the hypothalamic neuropeptides of PACAP (−/−) mice. Furthermore, we tried to overexpress PACAP selectively in the PACAP (+/+) VMH or PVH regions to investigate whether food intake behavior and expression of the neuropeptides were affected. Herein, our findings indicated that PACAP in the VMH facilitated food intake when mice craved food, which was at least partly due to upregulation of AgRP in the hypothalamus.
Materials and Methods
Animals
PACAP (−/−) mice had previously been generated and backcrossed into a CD-1 background [32]. All experiments were carried out with male littermates or CD-1 mice (Japan SLC Inc., Shizuoka, Japan) that were approximately 8–13 weeks old. All animals were singly housed under a standard 12-h light/dark cycle (light on at 7:00 a.m. and light off at 7:00 p.m.) for at least 1 week before and during experimentation. All experiments in the present study were approved by the Experimental Animal Research Committee of Kagoshima University (Approval numbers: MD17054 and MD18105) and the Gene Recombination Experiment Safety Committee of Kagoshima University (Approval number: S28006).
Strategy for PACAP Genotyping
The genotyping procedure was described previously [32]. Mouse ear punches (0.25 cm) were collected and incubated in 50 mM NaOH at 95 °C for 1 h. Subsequently, samples were neutralized by 1 M Tris-HCl (pH = 8.0). The PCR was performed in a thermal cycler (PC-320, ASTEC, Fukuoka, Japan) with an initial incubation at 95 °C for 3 min; 40 cycles of 95 °C for 30 s (denaturation), 59 °C for 30 s (annealing), and 72 °C for 2 min (extension), followed by holding at 4 °C. PCR products were visualized by electrophoresis in 1% agarose gels. Primers used for genotyping are shown in Supplemental file: Table S1.
Food Intake Evaluation
Food consumption was measured as described elsewhere [33]. Feeding studies were performed in home cages with ad libitum access to food and water. Diurnal, nocturnal, and daily food intake were assessed. For the fasting–refeeding paradigm, mice were fasted for 2 days and then refed at the indicated times. The body weight and food intake of mice were measured at 10 a.m before and 2 days after fasting, and 4, 8 and 24 h after refeeding.
i.c.v. Injection of PACAP6–38 and VIP6–28
PACAP6–38 was initially dissolved in 0.8% acetic acid (AcOH), aliquoted, and frozen until use. On the day of the experiment, aliquoted PACAP6–38 solution and 0.8% AcOH (as vehicle) were diluted in saline.
VIP6–28 solutions were made up as concentrated stock solutions in saline and used at the same doses as PACAP6–38.
Animals were fasted for 2 days, and PACAP (+/+) mice received an intracerebroventricular (i.c.v.) injection of either vehicle, PACAP6–38 (1 nmol) or VIP6–28 (1 nmol) 30 min before the start of refeeding at the coordinates: lateral (left), 1.8 mm; posterior, 0.5 mm from bregma, and 2.7 mm from the skull surface. Subsequently, either food intake or body weight was monitored at 1, 2, 4, and 24 h, or hypothalami were harvested 4 h after the i.c.v. injection.
Tissue Collection
After the fasting–refeeding experiments, mice were decapitated, and the PVH and VMH regions of hypothalamic were harvested from either naïve mice, mice fasted for 2 days, or mice refed for 4 h after being fasted for 2 days by forceps from coronal slices − 0.34 ~ − 3.34 mm, − 0.34 ~ − 1.34 mm, and − 1.34~− 2.34 mm from bregma. All samples were snap frozen in liquid nitrogen and kept at − 80 °C until use.
Mesenteric and epididymal tissues were isolated and weighed according to previous reports [17].
Assessment of Gene Expression by RT-qPCR
Total RNA was extracted using Sepasol-RNA 1 Super G kit (Nacalai Tesque, Kyoto, Japan), complementary DNA (cDNA) was generated using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA), and qPCR was carried out using a Thunderbird SYBR qPCR kit (Toyobo Life Science, Osaka, Japan) in a Thermal Cycler Dice (Real-Time System TP800, Takara Bio Inc., Shiga, Japan) with the use of specific primer sets for AgRP, POMC, NPY, CART, steroidogenic factor 1 (SF-1), PACAP, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Supplemental file: Table S2), according to the manufacturer’s protocol. The level of gene expression for each gene was normalized by GAPDH levels and expressed as relative expression units (%).
In Situ Hybridization
In situ hybridization was achieved as previously described [34]. Briefly, a partial cDNA fragment of mouse PACAP (Adcyap1 gene), amplified by RT-PCR, was cloned into a pTAC-2 vector (BioDynamics, Japan), and the resultant plasmid was used as a template for the synthesis of digoxigenin (DIG)-labeled antisense riboprobe. For cloning of the Adcyap1 cDNA fragment, the following primer set was used: 5′-TGGGTGCACAAGGATTGA-3′ and 5′-GGGCAAGGGTAGGAAGGA-3′. After fasting for 2 days, the animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneal injection (i.p.)) and transcardially perfused with heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Subsequently, the mouse brain was excised and post-fixed in the same fixative at 4 °C overnight and placed into 30% sucrose in 0.1 M phosphate-buffered saline (PBS) for cryoprotection at 4 °C. Mouse brain sections (30 μm) were cut on a cryostat (CM1850, Leica Microsystems, Nussloch, Germany), and collected sections were kept at − 30 °C until use. Next, specimens were treated with Triton X-100, proteinase K, acetic anhydride, and subsequently incubated in hybridization buffer with a DIG-labeled RNA probe against PACAP at 60 °C overnight. Specimens were then washed, incubated with RNAse to degrade non-hybridized probes, and further incubated with anti-DIG antibody-labeled alkaline phosphatase (AP) (1:3000, Roche Diagnostics, Mannheim, Germany) in blocking reagent at 4 °C overnight with a gentle shake. Samples were visualized by fast red TR/HNPP (Roche), counterstained with Hoechst 33342 (1:4000, Dojindo, Kumamoto, Japan), and mounted on silane-coated glass slides (Matsunami, Tokyo, Japan) with cc/mount (Diagnostic BioSystems, CA). Stained sections were examined under an all-in-one fluorescence microscope (BZ-X710, Keyence Co., Osaka, Japan).
Immunohistochemistry
Immunohistochemistry was carried out as previously described with slight modifications [35]. Brain slices were blocked with a blocking buffer containing 5% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO) and 0.3% Triton X-100 in PBS and incubated with the primary antibody against green fluorescent protein (#598, 1:1000, MBL, Nagoya, Japan) at 4 °C overnight. The sections were then incubated with Alexa Fluor® 488-labeled donkey anti-rabbit IgG antibody (1: 1000, Invitrogen, Carlsbad, CA) in 1% BSA at room temperature while being continuously shaken for 1 h. Samples were counterstained with Hoechst 33342 and mounted on silane-coated glass slides with cc/mount. Stained sections were visualized under BZ-X710.
Construction of AAV Vector for Gene Transfer
The virus was obtained as aforementioned with slight modifications [36]. Briefly, the coding region of mouse PACAP was amplified by the primer set, consisting of sequences 5′-ATGACCATGTGTAGCGGAGC-3′ and 5′-CTACAAGTATGCTATTCGGCGTC-3′ and cloned into an pAAV-CAG::IRES-EGFP plasmid to generate pAAV-CAG::PACAP-IRES-EGFP. AAV vectors (serotype 5) were produced by the AAVPro® Helper Free System (Takara). After mixing same amount of plasmid DNAs (pAAV, pRC5 and pHelper), the mixture of plasmids was transiently transfected into HEK293T cells using 1 mg/mL polyethyleneimine (Polyscience, Eppelheim, Germany) according to the manufacturer’s instructions. Three days after transfection, the HEK293T cells were harvested and lysed in PBS by freeze/thaw cycles. Cell lysates was treated with benzonase (Merck Millipore, Darmstadt, Germany) to degrade contaminated DNA and RNA, and subsequently, Opti-prep (Abbott Diagnostics Technologies AS, Oslo, Norway) gradient ultracentrifugation was used to purify AAV virions. The AAV virions were then concentrated using Vivaspin® 20 (Sartorius, Tokyo, Japan), and AAV titers was quantified as previously described [37]. The samples were then aliquoted and kept at − 80 °C until use.
Stereotaxic Injection
This method was implemented as previously described [38, 39]. Mice were anesthetized by i.p. injection of the mixture consisting of 0.75 mg/kg body weight (b.w.) medetomidine (Domitor® Nippon Zenyaku Kogyo Co., Ltd., Tokyo, Japan), 4.0 mg/kg b.w. midazolam (Dormicum®, Astellas Pharma Inc., Tokyo, Japan) and 5.0 mg/kg b.w. butorphanol (Vetorphale®, Meiji Seika Kaisha, Ltd., Tokyo, Japan) [40]. Holes were drilled in the skull, and 32G stainless needles were stereotaxically introduced into the appropriate positions, and AAVs (2 × 1013 viral genomes/mL) were bilaterally injected at a rate of 0.1 μL/min over 10 min. When the injection was completed, the needle was settled at this position for 5 min to diffuse the viral vector and then gently raised. The coordinates of specific brain regions were as follows: lateral, ± 0.4 mm; posterior, − 0.8 mm from the bregma and − 4.4 mm from the brain surface for PVH and lateral, ± 0.4 mm; posterior, − 1.4 mm from the bregma and − 4.8 mm from the brain surface for VMH [41]. Subsequently, the skin was sutured, and mice were injected with 0.75 mg/kg b.w. atipamezole to awaken them (Wako Pure Chemical Industries Ltd., Osaka, Japan) and 0.8 mg/mouse of penicillin G to prevent infections (Wako). Three weeks later, behavioral tests, body weight, and food intake were monitored at the indicated times.
Gene Transfection
pAAV-CAG::PACAP-IRES-EGFP plasmid was transiently transfected into HEK293T cells using polyethyleneimine (Polyscience) according to the manufacturer’s instructions. Two days later, total RNA isolation and immunocytochemistry were carried out.
Immunocytochemistry
The method in this study follows the protocol represented in a previous paper [42]. In brief, cells were plated on a glass cover slip (Matsunami) coated with collagen type 1. Samples were fixed with 4% paraformaldehyde in PB for 15 min, washed with PBS, and subsequently treated with 5% BSA and 0.3% Triton X-100 in PBS for 1 h. Cells were then incubated with a primary antibody against chicken anti-PACAP (1:500) diluted with 1% BSA in PBS at 4 °C overnight [43]. Staining was visualized with anti-chicken IgG conjugated to Alexa 647 (1:1000, Jackson ImmunoResearch, West Grove, PA). Samples were counterstained with Hoechst 33342 and mounted on silane-coated glass slides with cc/mount. Stained sections were examined with BZ-X710.
Open-Field Test
The open-field test was carried out as previously described [44]. Each mouse was placed in the center of an open-field apparatus (50 cm × 50 cm × 40 cm) and allowed to move freely for 5 min. Total travel distance and time spent in the center zone were measured using a video capture system (CompACT VAS, Muromachi Kikai, Tokyo, Japan).
Elevated Plus-Maze Test
The elevated plus-maze test was carried out as aforementioned [44, 45]. The plus-maze apparatus is made of gray plastic material consisting of two closed arms (25 cm × 5 cm × 15 cm), two open arms (25 cm × 5 cm × 0.5 cm), and the center square. The arms and the center square were placed at the 50-cm height from the floor. Each mouse was transferred to the center square and allowed to move freely in the maze for 5 min. The time spent on the open arms was measured using the video capture system (CompACT VAS).
Statistical Analyses
Results are shown as means ± S.E.M. A Student’s t test or analysis of variance (ANOVA) was used to assess statistical significance, followed by a Dunnett’s post-hoc test or Bonferroni’s multiple comparison test performed in Prism 4.3 software (Graph Pad, San Diego, CA). A P value under 0.05 was considered significant.
Results
PACAP (−/−) Mice Showed Reduced Food Intake Behavior
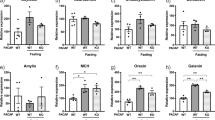
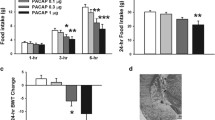
In order to reevaluate the functional importance of endogenous PACAP in appetite regulation, two paradigms of food intake measurement were applied to both PACAP (+/+) and (−/−) mice. Body weight (Fig. 1a) and food intake (Fig. 1b) in (−/−) mice were significantly decreased at 8 h after refeeding. However, initial body weights of these two genotypes remained unchanged (Fig. 1c), even though the fat masses of mesenteric and epidymal tissues in (−/−) were slightly lower than in (+/+) mice (Supplemental file: Fig. S1). In addition, food intake of PACAP (−/−) mice showed a tendency to increase in the diurnal period as compared to that of PACAP (+/+) mice (Fig. 1d). By contrast, nocturnal (Fig. 1e), and daily (Fig. 1f) food intake were significantly decreased in the (−/−) mice compared with (+/+) mice. With regard to the possible developmental effects of PACAP ablation, PACAP6–38, a PAC1 and VPAC2 receptor antagonist, was i.c.v.- injected into (+/+) mice 30 min before the start of the refeeding period. The injection of PACAP6–38 significantly decreased body weight (Fig. 1g) and food intake (Fig. 1h) at 1, 2, and 4 h time points after refeeding. Moreover, no difference in body weight or food intake was observed after i.c.v. administration of VIP6–28, a VPAC1 and VPAC2 receptor antagonist (Fig. 1i, j). These results suggested that endogenous PACAP might enhance appetite via PAC1 receptor signaling.
Blocking PACAP signaling reduced food intake. Changes in body weight (a) and cumulative food intake (b) of PACAP (+/+) and PACAP (−/−) mice at the indicated time after refeeding in mice who had already fasted for 2 days (n = 6 to 23 mice per genotype). Body weight (c) and food intake in diurnal (d) and nocturnal (e) periods and throughout the whole day (f) in (+/+) and (−/−) mice (n = 12 to 14 mice per genotype). Changes in body weight (g, i) and cumulative food intake (h, j) after i.c.v. injection of PACAP6–38 (1 nmol; g, h) or VIP6–28 (1 nmol; i, j) into (+/+) mice after refeeding in mice having already fasted for 2 days (n = 6 mice per group (PACAP6–38) and n = 3 mice per group (VIP6–28)). *p < 0.05; Student’s t test
Contribution of Endogenous PACAP to the Expression of Hypothalamic Neuropeptides
If PACAP contributes to appetite regulation, PACAP expression might be modified by fasting and refeeding. Hypothalamic PACAP mRNA expression was significantly increased by fasting for 2 days, and this expression returned to naïve levels after 4 h of refeeding (Fig. 2a). As expected, no PACAP expression was detected in (−/−) mice (Fig. 2a).
Contribution of endogenous PACAP to mRNA expression levels of agouti-related peptide (AgRP) and proopiomelanocortin (POMC) in the mouse hypothalamus after refeeding. Gene expression of PACAP (a), AgRP (b), NPY (c), CART (d), and POMC (e) was quantified by RT-qPCR analysis in the hypothalamus obtained from PACAP (+/+) (white bar) and (−/−) (black bar) mice, who are naive, have fasted for 2 days, and been refed for 4 h (n = 4 to 20 mice per group). Gene expression levels of AgRP, NPY, CART, and POMC (f) were measured in the hypothalamus of (+/+) mice 4 h after refeeding with or without i.c.v. injection of PACAP6–38 (1 nmol) 30 min before refeeding (n = 3 mice per group). *p < 0.05, **p < 0.01, ***p < 0.001; one-way ANOVA followed by Dunnett’s post-hoc test or Bonferroni’s multiple comparisons test.
Appetite is well known to be regulated by hypothalamic neuropeptides such as AgRP, NPY, CART and POMC [18,19,20]. Since food intake of (+/+) mice after refeeding was increased even at 8 h after refeeding, it is suggested that mRNA levels of feeding-related peptides would reflect their neural activity at 4 h after refeeding. Although AgRP and NPY expression levels were significantly upregulated by fasting and refeeding in both (+/+) and (−/−) mice (Fig. 2b, c), the magnitude of AgRP expression was significantly reduced in the (−/−) mice rather than in the (+/+) mice 4 h after refeeding (Fig. 2b). In contrast, although CART expression remained unaffected (Fig. 2d), POMC expression was significantly downregulated by fasting and refeeding in both (+/+) and (−/−) mice (Fig. 2e), but the magnitude of the downregulation was significantly less pronounced in the (−/−) mice than in the (+/+) mice 4 h after refeeding (Fig. 2e). Moreover, AgRP expression was also significantly reduced in mice injected with PACAP6–38 compared with mice injected with vehicle 4 h after refeeding, without any effect on the expression levels of NPY, CART, or POMC (Fig. 2f). Thus, these results suggested that PACAP might influence the expression levels of hypothalamic AgRP and POMC and that these expression changes might modulate appetite.
Fasting Increased Expression Levels of PACAP mRNA in the VMH Region
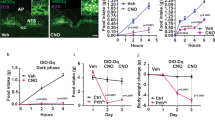
It had been previously reported that PACAP neurons located in both VMH and PVH regions send axonal projections to the ARC region of the hypothalamus and that both of these axonal projection tracts are considered to be involved in appetite regulation [13, 46]. Thus, we measured PACAP mRNA levels in both VMH and PVH regions after fasting and refeeding. PACAP mRNA expression in the VMH was significantly increased after fasting for 2 days without a significant effect on expression levels in PVH (Fig. 3a). SF-1, a VMH region marker [47], was only expressed in VMH samples, which confirmed that only the VMH region was being assessed, as desired (Fig. 3b). Likewise, in situ hybridization clearly demonstrated enhanced expression of PACAP mRNA in the cells of the VMH region (Fig. 3c). Thus, 2-day fasting selectively upregulated PACAP expression in the VMH region without affecting expression in the PVH region.
PACAP mRNA expression levels were enhanced in the ventromedial hypothalamus (VMH) region rather than the paraventricular hypothalamus (PVH) region after 2 days of fasting. PACAP (a) and SF-1 (b), a marker of the VMH region; mRNA expression levels in the PVH and VMH were measured by RT-qPCR (n = 8 to 10 mice per group). PACAP-expressing cells in the VMH region were visualized by in situ hybridization after fasting (c). Scale bars, 100 μm. **p < 0.01; one-way ANOVA followed by Dunnett’s post-hoc test
Enhancement of Food Intake After Selective Overexpression of PACAP in the VMH Region
Since PACAP expression in the VMH region was significantly increased by fasting, we speculated that increment of PACAP in the VMH would exert an orexigenic effect. To assess this possibility, we bilaterally injected the AAV vector carrying PACAP (PACAPVMH) or EGFP (EGFPVMH) into the VMH region (Fig. 4a, b). PACAP expression from the original plasmid was validated by RT-qPCR (Supplemental file: Fig. S2a) and immunocytochemistry (Supplemental file: Fig. S2b), in which PACAP-immunoreactive signals were only observed in EGFP-expressing cells after transfection into HEK293T cells. We found that the distance travelled (Fig. 4c) and the time spent in the center region (Fig. 4d) in the open-field test were not significantly different between EGFPVMH and PACAPVMH, suggesting that basal activity remained unchanged by PACAP overexpression in the VMH region. Although no effect was observed on diurnal food intake (Fig. 4e), the food intake in the nocturnal period was significantly increased in PACAPVMH mice rather than in the EGFPVMH mice (Fig. 4f). As a result, body weight gain in PACAPVMH mice was more pronounced than in EGFPVMH mice during the 3 weeks following the virus infection (Fig. 4g). Furthermore, we also observed that food intake at 8 h after refeeding was significantly increased in PACAPVMH mice rather than in EGFPVMH mice (Fig. 4h). These results suggested that PACAP in the VMH region had an orexigenic effect during the nocturnal period and after fasting.
Selective overexpression of PACAP in the VMH region increased food intake. a Simplified plasmid maps which were packaged into an adeno-associated virus (AAV) and a schematic showing bilateral VMH injection of the AAV virions. b Representative mouse brain section infected with an AAV expressing CAG::PACAP-IRES-EGFP in the VMH region. Scale bars, 100 μm. The distance travelled (c) and the time spent in the center region (d) in mice infected with an AAV expressing EGFP or PACAP in the VMH region (EGFPVMH or PACAPVMH). Food intake of EGFPVMH or PACAPVMH in diurnal (e) and nocturnal periods (f) was measured. Changes in body weight at 3 weeks after intra-VMH injections in EGFPVMH and PACAPVMH mice (g) and cumulative food intake were measured (h) 4, 8, and 24 h after refeeding in EGFPVMH and PACAPVMH mice, which had already fasted for 2 days (n = 6 mice per group). *p < 0.05, **p < 0.01; Student’s t test
Overexpression of PACAP in the VMH Induced a Time-Dependent Increase in the Expression Levels of POMC (Diurnal Period) and AgRP (Nocturnal Period)
To further explore expression changes in hypothalamic neuropeptides after the overexpression of PACAP in the VMH, we obtained hypothalamic samples in diurnal and nocturnal periods. The PACAP mRNA expression in the hypothalamus was increased in PACAPVMH mice than EGFPVMH mice in both diurnal and nocturnal periods (Fig. 5a, b). In the diurnal period, POMC expression was significantly increased in PACAPVMH mice rather than in EGFPVMH mice, without affecting the expression of AgRP, NPY or CART (Fig. 5a). In contrast, in the nocturnal period, AgRP expression was significantly increased in PACAPVMH mice rather than in EGFPVMH mice, without affecting the expression of NPY, CART, or POMC (Fig. 5b). It is worth noting that food intake was significantly upregulated in the nocturnal period, and tended to decrease in the diurnal period in PACAPVMH mice (see Fig. 4e and f). Differential upregulation of POMC and AgRP peptides in the diurnal and nocturnal periods appeared to be correlated to differential feeding behaviors in these periods in PACAPVMH mice.
Selective overexpression of PACAP in the VMH region changed the expression levels of POMC and AgRP in a time-dependent manner. Gene expression levels of PACAP, AgRP, NPY, CART, and POMC in diurnal (a) and nocturnal periods (b) were quantified by RT-qPCR (n = 3 mice per period). **p < 0.01, ***p < 0.001; Student’s t test
Selective Overexpression of PACAP in the PVH Region Did Not Change Food Intake
To examine the functional relevance of PACAP in the PVH region of the hypothalamus, we also bilaterally injected an AAV expressing PACAP (PACAPPVH) or EGFP (EGFPPVH) into the PVH region (Fig. 6a, b). In the open-field test, both the distance travelled (Fig. 6c) and the time spent in the center region (Fig. 6d) were decreased in PACAPPVH mice compared with EGFPPVH mice. Consistent with these results, we also observed that (−/−) mice showed enhanced locomotor activity in the open-field test and spent longer in the open arm in the elevated plus-maze test, although the time spent in the center region was comparable between (+/+) and (−/−) mice in the open-field test (Supplemental file: Fig. S3). However, no significant changes in food intake were observed between PACAPPVH and EGFPPVH mice during diurnal (Fig. 6e) and nocturnal periods (Fig. 6f). These results suggested that PACAP in the PVH would not play a significant role in food intake behavior.
Selective overexpression of PACAP in the PVH region increased anxiety-like behaviors without affecting food intake. a Schematic drawing bilateral PVH injections of the AAV expressing PACAP or control EGFP. b Representative mouse brain section infected with an AAV expressing CAG::PACAP-IRES-EGFP in the PVH region. Scale bars, 100 μm. Distance travelled (c) and time spent in the center region (d) were examined by the open-field test in mice infected with an AAV expressing EGFP or PACAP in the PVH region (EGFPPVH or PACAPPVH). Diurnal (e) and nocturnal (f) food intake were examined in EGFPPVH and PACAPPVH mice (n = 3 mice per group). *p < 0.05; Student’s t test
Discussion
To the best of our knowledge, this study is the first to identify the mechanism underlying decreased food intake in PACAP (−/−) mice. Specifically, we showed that endogenous PACAP might work via PAC1 receptors to enhance food intake behavior. In addition, PACAP may influence expression levels of hypothalamic neuropeptides including AgRP and POMC, known to play important roles in appetite regulation. Finally, we demonstrated that PACAP in the VMH region had an orexigenic effect during the nocturnal period and after fasting.
In the present study, we showed that the food intake at the 8 h time point after refeeding was significantly lower in PACAP (−/−) mice than in (+/+) mice. Nocturnal and daily food intake of PACAP (−/−) mice was also significantly decreased compared to (+/+) mice. In addition, i.c.v. administration of the PACAP receptor antagonist, PACAP6–38, decreased food intake and body weight gain in (+/+) mice 1, 2, and 4 h after refeeding. These observations suggested that endogenous PACAP may enhance appetite. Therefore, blocking PACAP signaling might be a potential strategy for developing novel drugs for the control of body weight and appetite regulation.
Interestingly, PACAP control of food intake behavior appeared to be state-dependent. In the nocturnal period, food consumption was significantly attenuated in PACAP (−/−) mice but was slightly increased in the diurnal period compared to PACAP (+/+) mice. Conversely, food consumption in the nocturnal period was significantly increased in PACAPVMH mice but was slightly decreased in the diurnal period compared to EGFPVMH mice. Likewise, orexigenic AgRP expression was significantly increased in the nocturnal period, but anorexigenic POMC expression was significantly increased in the diurnal period in PACAPVMH mice compared to EGFPVMH mice. These results suggested that PACAP in the VMH region may stimulate appetite in the nocturnal period when mice craved food. Our observation may be in line with a recent report demonstrating that PACAP-containing neurons co-expressed glutamate [48] and that glutamatergic afferents onto NPY/AgRP or CART/POMC neurons, which would be at least partly expected to contain PACAP, were rewired depending on metabolic state. In further detail, during states of negative energy balance when levels of circulating leptin are low, PACAPergic/glutamatergic afferents preferentially connect with NPY/AgRP neurons [48]. This may explain the specific modulation of NPY and AgRP expression during the nocturnal period. On the other hand, PACAPergic/glutamatergic afferents preferentially connect with CART/POMC neurons in states of satiety during the diurnal period, thereby activating the anorexigenic circuit [48]. Therefore, this is the first study to elucidate the role of PACAP signaling in the VMH region in appetite regulation.
The present study also suggested that PACAP might enhance appetite via PAC1 receptors, since PACAP ablation and injection of PACAP6–38 (the PAC1 and VPAC2 receptors antagonist), but not VIP6–28 (the VPAC1 and VPAC2 receptors antagonist), significantly decreased food intake in fasted mice. In support of our study, several previous studies have shown that AgRP neurons indeed express PAC1 receptors [16, 29, 31]. A recent study also reported that activation of PAC1 receptors expressed on AgRP neurons promoted food intake [29]. Thus, PAC1 receptors seem to play an important role in appetite regulation.
PACAP is highly expressed in hypothalamic regions that centrally regulate appetite [4]. Our results indicate that PACAP gene expression in the hypothalamus significantly increased after fasting. Therefore, we wondered whether an increase in PACAP expression would also be strongly associated with the expression of hypothalamic neuropeptides AgRP, NPY, CART, and POMC, well known to be critical for the regulation of feeding in the ARC region [18,19,20]. In support of this notion, we found that fasting and refeeding significantly increased the AgRP gene expression but decreased that of POMC in the hypothalamus. Furthermore, AgRP expression levels were significantly less increased but those of POMC were less decreased after refeeding in (−/−) mice compared to (+/+) mice. Moreover, our findings showed that AgRP expression levels were dramatically attenuated by i.c.v. injection of PACAP6–38 at 4 h after refeeding, although POMC expression remained unchanged by the PACAP6–38 injection. At this time, we do not know the precise reason for which POMC expression remained unchanged when PACAP6–38 was injected. It is likely that the use of the fasting–refeeding paradigm may explain these results. A previous study reported that POMC cells of the ARC express both PAC1 and VPAC2 receptors to induce a state of satiety [9]. However, these previous studies have demonstrated that PAC1 receptors were expressed on AgRP neurons to induce states of food craving and hunger [29]. Therefore, in our fasting–refeeding models, PACAP6–38 injection may work via high affinity PAC1 receptor-binding on AgRP neurons. Taken together, these findings suggested that PACAP may play a more important role in enhancing AgRP expression via fasting-induced orexigenic signals. The activation of AgRP neurons via PAC1 receptors induced the expression of a transcription factor, KLF4, which would trigger the increase of AgRP expression to boost food intake [29]. This mechanism may be involved in increased AgRP expression in PACAPVMH during the nocturnal period.
In general, PACAP has been considered an anorexigenic neuropeptide because the series of intrabrain injections of PACAP into PVH, VMH, CeA, and BNST all reduced food intake [9, 10, 14, 15]. Therefore, these previous studies suggested that exogenous PACAP had an anorexigenic effect. However, recent work has revealed that PACAP-expressing neurons from VMH and PVH activated both POMC-expressing anorexigenic neurons and AgRP-expressing orexigenic neurons [31]. In particular, activation of PACAPergic neurons by designer receptors exclusively activated by designer drugs (DREADDs) in the PVH induced an orexigenic effect, which was significantly suppressed by the injection of PACAP6–38, suggesting that PACAPergic neurons in PVH are orexigenic [29]. Although all of this work appears to contradict our results, nearly all studies were carried out in the diurnal period. Indeed, our overexpression of PACAP in the VMH also showed a considerable anorexigenic effect on food intake and significant upregulation of anorexigenic POMC expression in the diurnal period. Thus, although further studies are necessary to clarify the role of hypothalamic PACAP function on food intake, we propose here that VMH PACAPergic neurons have an orexigenic function at least during the nocturnal period.
An earlier report suggested that PACAP mRNA and protein expression within the VMH were elevated in high fat diets and decreased in fasted mice [49]. However, our study demonstrated that PACAP mRNA within the VMH was increased in fasted mice (Fig. 3a, b). We convinced our results were reliable because PACAP mRNA expression is not detected in (−/−) mice in the same qPCR experiment. Moreover, the qPCR results were supported by the ISH studies using mRNA probes for PACAP (Fig. 3c). By using a Cre-dependent AAV in Cre-driver mice to target specific populations of neurons (POMC, AgRP, NPY), further experiments to elucidate the detailed underlying mechanisms including the upstream partners of PACAP-containing neural circuits could contribute to resolving this contradiction.
Previous studies have shown that PACAP (−/−) mice exhibited less anxiety-like behaviors and higher locomotor activity [32, 50]. Likewise, a single i.c.v. injection of PACAP induced anxiety-like behaviors in the elevated plus-maze test [51]. The present study complements and extends these previous studies by providing data on the effects of selective overexpression of PACAP in the PVH region evaluated by the open-field test (Fig. 6). Interestingly, we showed that the PACAPPVH mice decreased the time spent in the center region and the distance travelled in the open-field test, suggesting that PACAP signaling in the PVH might be involved in anxiety-related behaviors. A recent study has demonstrated the impairment of auditory function in PACAP (−/−) mice [7]. However, auditory function may not play an important role regarding the above-mentioned behavioral alterations, because selective overexpression of PACAP in PVH showed the anxiety-like behavior. Further evaluation is needed to understand the functional significance of PACAP in the PVH region in order to establish the link to anxiety-like behavior.
The epididymal and mesenteric fat were decreased in PACAP (−/−) mice, suggesting that adipose browning may change. Previous study suggested the genes related to adipose browning such as peroxisome proliferator-activated receptor gamma coactivator 1-alpha and uncoupling protein 1 were significantly decreased in PACAP (−/−) mice [52]. As a future study, it should be interesting to clarify the relationship between PACAP and adipose browning.
Taken together, the present study’s results demonstrate that VMH PACAP controls food intake behavior by modulating AgRP expression in the mouse hypothalamus in the nocturnal periods and that mechanism may play a role in enhancing food motivation. Targeting PACAP signaling, therefore, could be a clinically important therapeutic strategy for body weight control and appetite regulation.
Abbreviations
- AgRP:
-
Agouti-related peptide
- BNST:
-
Bed nucleus of the stria terminalis
- CeA:
-
Central amygdala
- CART:
-
Cocaine- and amphetamine-regulated transcript
- NPY:
-
Neuropeptide Y
- SF-1:
-
Steroidogenic factor 1
- PACAP:
-
Pituitary adenylate cyclase-activating polypeptide
- POMC:
-
Proopiomelanocortin
- PVH:
-
Paraventricular hypothalamus
- VMH:
-
Ventromedial hypothalamus
- (−/−):
-
Knockout
- (+/+):
-
Wild type
References
Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164(1):567–574. https://doi.org/10.1016/0006-291X(89)91757-9
Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A (1990) Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun 170(2):643–648. https://doi.org/10.1016/0006-291X(90)92140-U
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC et al (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61(3):283–357. https://doi.org/10.1124/pr.109.001370
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52(2):269–324
Yokai M, Kurihara T, Miyata A (2016) Spinal astrocytic activation contributes to both induction and maintenance of pituitary adenylate cyclase-activating polypeptide type 1 receptor-induced long-lasting mechanical allodynia in mice. Mol Pain 12:1744806916646383. https://doi.org/10.1177/1744806916646383
Rudecki AP, Gray SL (2016) PACAP in the defense of energy homeostasis. Trends Endocrinol Metab 27(9):620–632. https://doi.org/10.1016/j.tem.2016.04.008
Fulop DB, Humli V, Szepesy J, Ott V, Reglodi D, Gaszner B, Nemeth A, Szirmai A et al (2019) Hearing impairment and associated morphological changes in pituitary adenylate cyclase activating polypeptide (PACAP)-deficient mice. Sci Rep 9(1):14598. https://doi.org/10.1038/s41598-019-50775-z
Denes V, Geck P, Mester A, Gabriel R (2019) Pituitary adenylate cyclase-activating polypeptide: 30 years in research spotlight and 600 million years in service. J Clin Med 8(9):1488. https://doi.org/10.3390/jcm8091488
Mounien L, Bizet P, Boutelet I, Gourcerol G, Fournier A, Vaudry H, Jégou S (2006) Pituitary adenylate cyclase-activating polypeptide directly modulates the activity of proopiomelanocortin neurons in the rat arcuate nucleus. Neuroscience 143(1):155–163. https://doi.org/10.1016/j.neuroscience.2006.07.022
Mounien L, Do Rego J-C, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J et al (2008) Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology 34(2):424–435
Resch JM, Maunze B, Phillips KA, Choi S (2014) Inhibition of food intake by PACAP in the hypothalamic ventromedial nuclei is mediated by NMDA receptors. Physiol Behav 133:230–235. https://doi.org/10.1016/j.physbeh.2014.05.029
Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S (2011) Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Phys Regul Integr Comp Phys 301(6):R1625–R1634. https://doi.org/10.1152/ajpregu.00334.2011
Resch JM, Maunze B, Gerhardt AK, Magnuson SK, Phillips KA, Choi S (2013) Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am J Physiol Endocrinol Metab 305(12):E1452–E1463. https://doi.org/10.1152/ajpendo.00293.2013
Iemolo A, Ferragud A, Cottone P, Sabino V (2015) Pituitary adenylate cyclase-activating peptide in the central amygdala causes anorexia and body weight loss via the melanocortin and the TrkB systems. Neuropsychopharmacology 40(8):1846–1855. https://doi.org/10.1038/npp.2015.34
Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, Edwards S, Wasserman E et al (2014) PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology 39(7):1614–1623. https://doi.org/10.1038/npp.2014.8
Nakata M, Kohno D, Shintani N, Nemoto Y, Hashimoto H, Baba A, Yada T (2004) PACAP deficient mice display reduced carbohydrate intake and PACAP activates NPY-containing neurons in the rat hypothalamic arcuate nucleus. Neurosci Lett 370(2–3):252–256. https://doi.org/10.1016/j.neulet.2004.08.034
Tomimoto S, Ojika T, Shintani N, Hashimoto H, K-i H, Ikeda K, Nakata M, Yada T et al (2008) Markedly reduced white adipose tissue and increased insulin sensitivity in Adcyap1-deficient mice. J Pharmacol Sci 107(1):41–48. https://doi.org/10.1254/jphs.FP0072173
Hahn TM, Breininger JF, Baskin DG, Schwartz MW (1998) Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1:271–272. https://doi.org/10.1038/1082
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295. https://doi.org/10.1038/nature05026
Biebermann H, Kühnen P, Kleinau G, Krude H (2012) The neuroendocrine circuitry controlled by POMC, MSH, and AGRP. In: Joost H-G (ed) Appetite control. Springer, Berlin Heidelberg, pp. 47–75. https://doi.org/10.1007/978-3-642-24716-3_3
Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Ploeg LHTV, Woods SC, Seeley RJ (2000) Long-term orexigenic effects of AgRP-(83—132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol 279(1):R47–R52. https://doi.org/10.1152/ajpregu.2000.279.1.R47
Luquet S, Perez FA, Hnasko TS, Palmiter RD (2005) NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310(5748):683–685. https://doi.org/10.1126/science.1115524
Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411(6836):480–484. https://doi.org/10.1038/35078085
Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford MLJ (2000) Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3(8):757–758. https://doi.org/10.1038/77660
Air EL, Clegg DJ, Seeley RJ, Benoit SC, Woods SC (2002) Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology 143(6):2449–2452. https://doi.org/10.1210/endo.143.6.8948
Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M et al (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37(4):649–661. https://doi.org/10.1016/S0896-6273(03)00063-1
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409(6817):194–198. https://doi.org/10.1038/35051587
Wren AM, Murphy KG, Seal LJ, Cohen MA, Ghatei MA, Bloom SR, Dhillo WS, Brynes AE et al (2001) Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86(12):5992–5992. https://doi.org/10.1210/jcem.86.12.8111
K-i N, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S et al (2016) Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun 7:10268. https://doi.org/10.1038/ncomms10268
Mizuno Y, Kondo K, Terashima Y, Arima H, Murase T, Oiso Y (1998) Anorectic effect of pituitary adenylate cyclase activating polypeptide (PACAP) in rats: lack of evidence for involvement of hypothalamic neuropeptide gene expression. J Neuroendocrinol 10(8):611–616
Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H et al (2014) An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507:238–242. https://doi.org/10.1038/nature12956
Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, J-i M et al (2001) Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci 98(23):13355–13360. https://doi.org/10.1073/pnas.231094498
Tong Q, Ye C-P, Jones JE, Elmquist JK, Lowell BB (2008) Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11(9):998–1000
Tochitani S, Liang F, Watakabe A, Hashikawa T, Yamamori T (2001) The occ1 gene is preferentially expressed in the primary visual cortex in an activity-dependent manner: a pattern of gene expression related to the cytoarchitectonic area in adult macaque neocortex. Eur J Neurosci 13(2):297–307. https://doi.org/10.1046/j.0953-816X.2000.01390.x
An JJ, Gharami K, Liao G-Y, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR et al (2008) Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134(1):175–187. https://doi.org/10.1016/j.cell.2008.05.045
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ et al (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 6:973–985. https://doi.org/10.1038/sj.gt.3300938
Christine Aurnhammer MH, Muether N, Hausl M, Rauschhuber C, Huber I, Nitschko H, Busch U, Sing A et al (2012) Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods 23(1):18–28. https://doi.org/10.1089/hgtb.2011.034
Kong D, Tong Q, Ye C, Koda S, Fuller Patrick M, Krashes Michael J, Vong L, Ray Russell S et al (2012) GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151(3):645–657. https://doi.org/10.1016/j.cell.2012.09.020
Krashes MJ, Shah BP, Koda S, Lowell BB (2013) Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab 18(4):588–595. https://doi.org/10.1016/j.cmet.2013.09.009
Kirihara Y, Takechi M, Kurosaki K, Kobayashi Y, Kurosawa T (2013) Anesthetic effects of a mixture of medetomidine, midazolam and butorphanol in two strains of mice. Exp Anim 62(3):173–180. https://doi.org/10.1538/expanim.62.173
Paxinos G, Franklin K (2011) The mouse brain in stereotaxic coordinates, 2nd Edition. Academic Press, San Diego, California. ISBN: 0-12-547637-X.
Kambe Y, Miyata A (2016) Mitochondrial c-Fos may increase the vulnerability of Neuro2a cells to cellular stressors. J Mol Neurosci 59(1):106–112. https://doi.org/10.1007/s12031-015-0710-7
Nakamachi T, Kamata E, Tanigawa A, Konno N, Shioda S, Matsuda K (2018) Distribution of pituitary adenylate cyclase-activating polypeptide 2 in zebrafish brain. Peptides 103:40–47. https://doi.org/10.1016/j.peptides.2018.03.006
Saegusa H, Kurihara T, Zong S, Minowa O, A-a K, Han W, Matsuda Y, Yamanaka H et al (2000) Altered pain responses in mice lacking α1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci 97(11):6132–6137. https://doi.org/10.1073/pnas.100124197
Le H, Ahn BJ, Lee HS, Shin A, Chae S, Lee SY, Shin MW, Lee E-J et al (2017) Disruption of Ninjurin1 leads to repetitive and anxiety-like behaviors in mice. Mol Neurobiol 54(9):7353–7368. https://doi.org/10.1007/s12035-016-0207-6
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL et al (2011) Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121(4):1424–1428. https://doi.org/10.1172/jci46229
Parker KL (1998) The roles of steroidogenic factor 1 in endocrine development and function. Mol Cell Endocrinol 145 (1):15–20. https://doi.org/10.1016/S0303-7207(98)00164-6
Horvath TL, Diano S (2004) The floating blueprint of hypothalamic feeding circuits. Nat Rev Neurosci 5(8):662–667
Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM (2009) PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci 29(47):14828–14835. https://doi.org/10.1523/jneurosci.1526-09.2009
Mustafa T, Jiang SZ, Eiden AM, Weihe E, Thistlethwaite I, Eiden LE (2015) Impact of PACAP and PAC1 receptor deficiency on the neurochemical and behavioral effects of acute and chronic restraint stress in male C57BL/6 mice. Stress 18(4):408–418. https://doi.org/10.3109/10253890.2015.1025044
Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V (2013) CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology 38(11):2160–2169. https://doi.org/10.1038/npp.2013.113
Bruce AA, Sarah LG, Emma RI, Antonio CB, Antonio JV-P, Nancy MS (2008) Feeding and metabolism in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 149(4):1571–1580. https://doi.org/10.1210/en.2007-0515
Acknowledgments
We would like to thank Ms. Izumi Fujisima and Mr. Tetsuya Kawamura for their technical contribution and all the staff members of the Joint Research Laboratory and the Division of Laboratory Animal Sciences, Kagoshima University for their help with animal care and the use of the facilities. We are also grateful to the Ministry of Agriculture and Rural Development, Vietnam, for the doctoral scholarship to T.T.N.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (C), Japan Society for the Promotion of Science (JSPS) (JSPS KAKENHI Grant No. 17K08310, 17 K08599 and JP19K07121), a Grant-in-Aid for Scientific Research (B) (Grant No. JP17H03989), MEXT KAKENHI, (grant number JP18H05416), and AMED (grant No. JP19dm0107122h0004 and JP19dm0207061h0003).
Author information
Authors and Affiliations
Contributions
T.T.N carried out the experiments, performed statistical analysis, and drafted the manuscript. YK carried out the experiments, performed behavioral studies, and wrote the manuscript. TK and AM conceived of and participated in the design of the study and wrote the manuscript. TN, NS, and HH participated in the design of the study and reviewed the manuscript. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Specifically, all experiments in the present study were approved by the Experimental Animal Research Committee of Kagoshima University (Approval numbers: MD17054 and MD18105) and the Gene Recombination Experiment Safety Committee of Kagoshima University (Approval number: S28006).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
PACAP (-/-) mice had reduced mesenteric and epididymal fat masses. Fat mass in mesenteric (a) and epididymal tissues (b) under the conditions indicated on the horizontal axis (n = 9 to 11 mice per group). *p<0.05; Student’s t-test (PNG 97 kb)
ESM 2
Successful expression of PACAP after transient transfection of pAAV-CAG::PACAP-IRES-EGFP. (a) HEK293 cells were transiently transfected with pAAV-CAG::IRES-EGFP or pAAV-CAG::PACAP-IRES-EGFP, and mRNA expression levels of PACAP and GAPDH (reference) were quantified by RT-qPCR (n = 2 mice per group). (b) HEK293 cells were transiently transfected with pAAV-CAG::PACAP-IRES-EGFP, and expression of PACAP (red) and GFP (green) was confirmed by immunohistochemistry with each specific antibody. Arrow indicates PACAP immunoreactivity. Scale bars, 10 μm (PNG 601 kb)
ESM 3
PACAP (-/-) mice exhibited enhanced locomotor activity and anti-anxiety-like behaviors compared with their PACAP (+/+) littermates. Distance travelled (a) and time spent in the center region (b) of (+/+) and (-/-) mice in the open-field test. The time spent in the open arms (c) of (+/+) and (-/-) mice in the elevated plus-maze test (n = 5 to 6 mice per group). *p<0.05; Student’s t-test (PNG 104 kb)
ESM 4
List of primer sequences used for genotyping (DOCX 13 kb)
ESM 5
List of primer sequences used for RT-qPCR (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Nguyen, T.T., Kambe, Y., Kurihara, T. et al. Pituitary Adenylate Cyclase-Activating Polypeptide in the Ventromedial Hypothalamus Is Responsible for Food Intake Behavior by Modulating the Expression of Agouti-Related Peptide in Mice. Mol Neurobiol 57, 2101–2114 (2020). https://doi.org/10.1007/s12035-019-01864-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01864-7