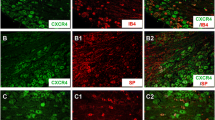
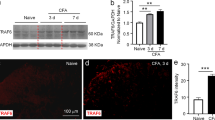
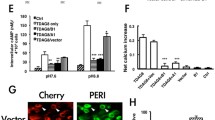
Abstract
Emerging evidence has demonstrated the involvement of stromal cell-derived factor 1 (SDF1, also known as CXCL12)-CXCR4 signaling in a variety of pain state. However, the underlying mechanisms of SDF1-CXCR4 signaling leading to the maintenance of chronic pain states are poorly understood. In the present study, we sought to explore the role of SDF1-CXCR4 signaling in the forming of neuroplasticity by applying a model of the transition from acute to chronic pain state, named as hyperalgesic priming. Utilizing intraplantar bee venom (BV) injection, we successfully established hyperalgesic priming state and found that peripheral treating with AMD3100, a CXCR4 antagonist, or knocking down CXCR4 by intraganglionar CXCR4 small interfering RNA (siRNA) injection could prevent BV-induced primary mechanical hyperalgesia and hyperalgesic priming. Moreover, we showed that single intraplantar active SDF1 protein injection is sufficient to induce acute mechanical hyperalgesia and hyperalgesic priming through CXC4. Intraplantar coinjection of ERK inhibitor, U0126, and PI3K inhibitor, LY294002, as well as two protein translation inhibitors, temsirolimus and cordycepin, prevented the development of SDF1-induced acute mechanical hyperalgesia and hyperalgesic priming. Finally, on the models of complete Freund’s adjuvant (CFA)-induced chronic inflammatory pain and spared nerve injury (SNI)-induced chronic neuropathic pain, we observed that knock-down of CXCR4 could both prevent the development and reverse the maintenance of chronic pain state. In conclusion, our present data suggested that through regulating ERK and PI3K-AKT pathways-mediated protein translation SDF1-CXCR4 signaling mediates the transition from acute pain to chronic pain state and finally contributes to the development and maintenance of chronic pain.








Similar content being viewed by others
References
Gaskin DJ, Richard P (2012) The economic costs of pain in the United States. J Pain 13:715–724
Jackson T, Thomas S, Stabile V, Han X, Shotwell M, McQueen K (2015) Prevalence of chronic pain in low-income and middle-income countries: a systematic review and meta-analysis. Lancet 385:S10
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R et al (2015) A classification of chronic pain for ICD-11. Pain 156:1003–1007
Luo X, Wang X, Xia Z, Chung SK, Cheung CW (2016) CXCL12/CXCR4 axis: an emerging neuromodulator in pathological pain. Rev Neurosci 27:83–92
Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J (2011) Spatiotemporal CCR1, CCL3(MIP-1alpha), CXCR4, CXCL12(SDF-1alpha) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J Neurosurg Spine 14:583–597
Dubovy P, Klusakova I, Svizenska I, Brazda V (2010) Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem Cell Biol 133:323–337
Bai L, Wang X, Li Z, Kong C, Zhao Y, Qian JL, Kan Q, Zhang W et al (2016) Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull 32:27–40
Menichella DM, Abdelhak B, Ren D, Shum A, Frietag C, Miller RJ (2014) CXCR4 chemokine receptor signaling mediates pain in diabetic neuropathy. Mol Pain 10:42
Shen W, Hu XM, Liu YN, Han Y, Chen LP, Wang CC, Song C (2014) CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 11:75
Hu XM, Liu YN, Zhang HL, Cao SB, Zhang T, Chen LP, Shen W (2015) CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem 132:452–463
Yang F, Sun W, Yang Y, Wang Y, Li CL, Fu H, Wang XL, Yang F et al (2015) SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation. J Neuroinflammation 12:219
Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F et al (2011) Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci 31:6646–6653
Wang H, Heijnen CJ, van Velthoven CT, Willemen HL, Ishikawa Y, Zhang X, Sood AK, Vroon A et al (2013) Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest 123:5023–5034
Ferrari LF, Bogen O, Reichling DB, Levine JD (2015) Accounting for the delay in the transition from acute to chronic pain: axonal and nuclear mechanisms. J Neurosci 35:495–507
Araldi D, Ferrari LF, Levine JD (2015) Repeated Mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci 35:12502–12517
Reichling DB, Levine JD (2009) Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 32:611–618
Aley KO, Messing RO, Mochly-Rosen D, Levine JD (2000) Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci 20:4680–4685
Melemedjian OK, Tillu DV, Asiedu MN, Mandell EK, Moy JK, Blute VM, Taylor CJ, Ghosh S et al (2013) BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain 9:12
Ferrari LF, Araldi D, Levine JD (2015) Distinct terminal and cell body mechanisms in the nociceptor mediate hyperalgesic priming. J Neurosci 35:6107–6116
Chen J, Lariviere WR (2010) The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword. Prog Neurobiol 92:151–183
Li Z, Gu X, Sun L, Wu S, Liang L, Cao J, Lutz BM, Bekker A et al (2015) Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain 156:711–721
Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158
Chen J, Luo C, Li H, Chen H (1999) Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain 83:67–76
Luo Y, Lathia J, Mughal M, Mattson MP (2008) SDF1alpha/CXCR4 signaling, via ERKs and the transcription factor Egr1, induces expression of a 67-kDa form of glutamic acid decarboxylase in embryonic hippocampal neurons. J Biol Chem 283:24789–24800
Badr G, Sayed A, Abdel-Maksoud MA, Mohamed AO, El-Amir A, Abdel-Ghaffar FA, Al-Quraishy S, Mahmoud MH (2015) Infection of female BWF1 lupus mice with malaria parasite attenuates B cell autoreactivity by modulating the CXCL12/CXCR4 axis and its downstream signals PI3K/AKT, NFkappaB and ERK. Plos One 10, e125340
Yang P, Wang G, Huo H, Li Q, Zhao Y, Liu Y (2015) SDF-1/CXCR4 signaling up-regulates survivin to regulate human sacral chondrosarcoma cell cycle and epithelial-mesenchymal transition via ERK and PI3K/AKT pathway. Med Oncol 32:377
Gilron I, Jensen TS, Dickenson AH (2013) Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol 12:1084–1095
Dieppe PA, Lohmander LS (2005) Pathogenesis and management of pain in osteoarthritis. Lancet 365:965–973
Tiveron MC, Cremer H (2008) CXCL12/CXCR4 signalling in neuronal cell migration. Curr Opin Neurobiol 18:237–244
Guyon A (2014) CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci 8:65
Chatterjee M, Rath D, Gawaz M (2015) Role of chemokine receptors CXCR4 and CXCR7 for platelet function. Biochem Soc Trans 43:720–726
Nagasawa T (2015) CXCL12/SDF-1 and CXCR4. Front Immunol 6:301
Wilson NM, Jung H, Ripsch MS, Miller RJ, White FA (2011) CXCR4 signaling mediates morphine-induced tactile hyperalgesia. Brain Behav Immun 25:565–573
Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ (2001) Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci 21:5027–5035
Ma W, Quirion R (2005) The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opin Ther Targets 9:699–713
Fang D, Kong LY, Cai J, Li S, Liu XD, Han JS, Xing GG (2015) Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain 156:1124–1144
Jiang SP, Zhang ZD, Kang LM, Wang QH, Zhang L, Chen HP (2016) Celecoxib reverts oxaliplatin-induced neuropathic pain through inhibiting PI3K/Akt2 pathway in the mouse dorsal root ganglion. Exp Neurol 275:11–16
Migliaccio N, Sanges C, Ruggiero I, Martucci NM, Rippa E, Arcari P, Lamberti A (2013) Raf kinases in signal transduction and interaction with translation machinery. Biomol Concepts 4:391–399
Ferrari LF, Bogen O, Chu C, Levine JD (2013) Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain 14:731–738
Kim MH, Nahm FS, Kim TK, Chang MJ, Do SH (2014) Comparison of postoperative pain in the first and second knee in staged bilateral total knee arthroplasty: clinical evidence of enhanced pain sensitivity after surgical injury. Pain 155:22–27
Hutchinson MR, Buijs M, Tuke J, Kwok YH, Gentgall M, Williams D, Rolan P (2013) Low-dose endotoxin potentiates capsaicin-induced pain in man: evidence for a pain neuroimmune connection. Brain Behav Immun 30:3–11
Ferrari LF, Bogen O, Levine JD (2013) Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci 33:11002–11011
Alvarez P, Green PG, Levine JD (2014) Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain 155:1161–1167
Kim JY, Tillu DV, Quinn TL, Mejia GL, Shy A, Asiedu MN, Murad E, Schumann AP et al (2015) Spinal dopaminergic projections control the transition to pathological pain plasticity via a D1/D5-mediated mechanism. J Neurosci 35:6307–6317
Araldi D, Ferrari LF, Levine JD (2015) Adenosine-A1 receptor agonist induced hyperalgesic priming type II. Pain 157(3):698–709
Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Nahon JL, Rostene W et al (2007) The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J Neurochem 102:1175–1183
Limatola C, Giovannelli A, Maggi L, Ragozzino D, Castellani L, Ciotti MT, Vacca F, Mercanti D et al (2000) SDF-1alpha-mediated modulation of synaptic transmission in rat cerebellum. Eur J Neurosci 12:2497–2504
Ardelt AA, Bhattacharyya BJ, Belmadani A, Ren D, Miller RJ (2013) Stromal derived growth factor-1 (CXCL12) modulates synaptic transmission to immature neurons during post-ischemic cerebral repair. Exp Neurol 248:246–253
Pujol F, Kitabgi P, Boudin H (2005) The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J Cell Sci 118:1071–1080
Melemedjian OK, Tillu DV, Moy JK, Asiedu MN, Mandell EK, Ghosh S, Dussor G, Price TJ (2014) Local translation and retrograde axonal transport of CREB regulates IL-6-induced nociceptive plasticity. Mol Pain 10:45
Thakor DK, Lin A, Matsuka Y, Meyer EM, Ruangsri S, Nishimura I, Spigelman I (2009) Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain 5:14
Acknowledgments
This work was supported by National Basic Research Development Program of China (2013CB835100), the National Natural Science Foundation of China (81171049, 81571072, 31300919, 31400948), and the National Key Technology R&D Program (2013BAI04B04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Fei Yang, Wei Sun and Wen-Jun Luo contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, F., Sun, W., Luo, WJ. et al. SDF1-CXCR4 Signaling Contributes to the Transition from Acute to Chronic Pain State. Mol Neurobiol 54, 2763–2775 (2017). https://doi.org/10.1007/s12035-016-9875-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9875-5