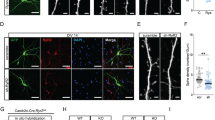
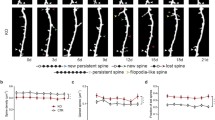
Abstract
Dendritic spines, a special kind of structure in nerve cells, play a key role in performing cellular function. Structural abnormalities of the dendritic spine may contribute to synaptic dysfunction and have been implicated in memory formation. However, the molecular mechanisms that trigger dendritic spine loss remain unclear. Here, we show that the absence of dendritic cell factor 1 (Dcf1) appeared dendritic spines dysplasia, which in turn leads to the damage of learning and memory; in contrast, enhancing Dcf1 expression rescues dendritic spines morphology and function, indicating a pivotal role of Dcf1 in cellular function. Electrophysiological test indicates that there is a significant reduction in the frequency of miniature excitatory postsynaptic currents in Dcf1−/− knockout (KO) mice. Subsequent to optogenetic ignition, we observed a weaker neuronal activation in Dcf1 KO mice, explaining the neural circuit cause. On molecular mechanism, we demonstrated an unprecedented discovery that Dcf1 triggers the dendritic spine and synaptic function through the recruitment of Lcn2 and activation of PSD95-NMDAR signaling. Removing this brake leads to memory damage. Our results highlight an unexpected regulatory mechanism of dendritic spine development and formation.







Similar content being viewed by others
References
Banerjee J, Fischer CC, Wedegaertner PB (2009) The amino acid motif L/IIxxFE defines a novel actin-binding sequence in PDZ-RhoGEF. Biochemistry 48(33):8032–8043. doi:10.1021/bi9010013
Tschida KA, Mooney R (2012) Deafening drives cell-type-specific changes to dendritic spines in a sensorimotor nucleus important to learned vocalizations. Neuron 73(5):1028–1039. doi:10.1016/j.neuron.2011.12.038
Bian WJ, Miao WY, He SJ, Qiu Z, Yu X (2015) Coordinated spine pruning and maturation mediated by inter-spine competition for cadherin/catenin complexes. Cell 162(4):808–822. doi:10.1016/j.cell.2015.07.018
Merino-Serrais P, Benavides-Piccione R, Blazquez-Llorca L, Kastanauskaite A, Rabano A, Avila J, DeFelipe J (2013) The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer's disease. Brain : a journal of neurology 136(Pt 6):1913–1928. doi:10.1093/brain/awt088
Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD et al (2009) Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 462(7276):1065–1069. doi:10.1038/nature08628
Kondratiuk I, Leski S, Urbanska M, Biecek P, Devijver H, Lechat B, Van Leuven F, Kaczmarek L et al (2016) GSK-3beta and MMP-9 cooperate in the control of dendritic spine morphology. Mol Neurobiol. doi:10.1007/s12035-015-9625-0
Chen Y, Wang F, Long H, Chen Y, Wu Z, Ma L (2011) GRK5 promotes F-actin bundling and targets bundles to membrane structures to control neuronal morphogenesis. J Cell Biol 194(6):905–920. doi:10.1083/jcb.201104114
Li J, Gu J, Wang B, Xie M, Huang L, Liu Y, Zhang L, Xue J et al (2015) Activation of dopamine D1 receptors regulates dendritic morphogenesis through Rac1 and RhoA in prefrontal cortex neurons. Mol Neurobiol 51(3):1024–1037. doi:10.1007/s12035-014-8762-1
Wen T, Gu P, Chen F (2002) Discovery of two novel functional genes from differentiation of neural stem cells in the striatum of the fetal rat. Neurosci Lett 329(1):101–105
Wang L, Wang J, Wu Y, Wu J, Pang S, Pan R, Wen T (2008) A novel function of dcf1 during the differentiation of neural stem cells in vitro. Cell Mol Neurobiol 28(6):887–894. doi:10.1007/s10571-008-9266-1
Li X, Feng R, Huang C, Wang H, Wang J, Zhang Z, Yan H, Wen T (2012) MicroRNA-351 regulates TMEM 59 (DCF1) expression and mediates neural stem cell morphogenesis. RNA Biol 9(3):292–301. doi:10.4161/rna.19100
Boada-Romero E, Letek M, Fleischer A, Pallauf K, Ramon-Barros C, Pimentel-Muinos FX (2013) TMEM59 defines a novel ATG16L1-binding motif that promotes local activation of LC3. EMBO J 32(4):566–582. doi:10.1038/emboj.2013.8
Zhao C, Teng EM, Summers RG Jr, Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 26(1):3–11. doi:10.1523/JNEUROSCI.3648-05.2006
Cubelos B, Sebastian-Serrano A, Beccari L, Calcagnotto ME, Cisneros E, Kim S, Dopazo A, Alvarez-Dolado M et al (2010) Cux1 and Cux2 regulate dendritic branching, spine morphology, and synapses of the upper layer neurons of the cortex. Neuron 66(4):523–535. doi:10.1016/j.neuron.2010.04.038
Ohnuma T, Kato H, Arai H, Faull RL, McKenna PJ, Emson PC (2000) Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport 11(14):3133–3137
Beresewicz M, Kowalczyk JE, Zablocka B (2008) Kalirin-7, a protein enriched in postsynaptic density, is involved in ischemic signal transduction. Neurochem Res 33(9):1789–1794. doi:10.1007/s11064-008-9631-y
Liu RX, Wang WQ, Ye L, Bi YF, Fang H, Cui B, Zhou WW, Dai M et al (2010) p21-activated kinase 3 is overexpressed in thymic neuroendocrine tumors (carcinoids) with ectopic ACTH syndrome and participates in cell migration. Endocrine 38(1):38–47. doi:10.1007/s12020-010-9324-6
Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC (2009) Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem 284(23):15857–15866. doi:10.1074/jbc.M808986200
Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT (2004) Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304(5673):1021–1024. doi:10.1126/science.1096615
Wang XD, Su YA, Wagner KV, Avrabos C, Scharf SH, Hartmann J, Wolf M, Liebl C et al (2013) Nectin-3 links CRHR1 signaling to stress-induced memory deficits and spine loss. Nat Neurosci 16(6):706–713. doi:10.1038/nn.3395
Snigdha S, Prieto GA, Petrosyan A, Loertscher BM, Dieskau AP, Overman LE, Cotman CW (2016) H3K9me3 inhibition improves memory, promotes spine formation, and increases BDNF levels in the aged hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 36(12):3611–3622. doi:10.1523/JNEUROSCI.2693-15.2016
Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP et al (2006) Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442(7104):814–817. doi:10.1038/nature04976
Flower DR (1996) The lipocalin protein family: structure and function. The Biochemical journal 318(Pt 1):1–14
Flower DR, North AC, Sansom CE (2000) The lipocalin protein family: structural and sequence overview. Biochim Biophys Acta 1482(1–2):9–24
Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H et al (2002) An iron delivery pathway mediated by a lipocalin. Mol Cell 10(5):1045–1056
Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R (1999) Developmental regulation of spine motility in the mammalian central nervous system. Proc Natl Acad Sci U S A 96(23):13438–13443
Palchykova S, Winsky-Sommerer R, Meerlo P, Durr R, Tobler I (2006) Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem 85(3):263–271. doi:10.1016/j.nlm.2005.11.005
Schmidt MV, Schulke JP, Liebl C, Stiess M, Avrabos C, Bock J, Wochnik GM, Davies HA et al (2011) Tumor suppressor down-regulated in renal cell carcinoma 1 (DRR1) is a stress-induced actin bundling factor that modulates synaptic efficacy and cognition. Proc Natl Acad Sci U S A 108(41):17213–17218. doi:10.1073/pnas.1103318108
Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W et al (2011) Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. The Journal of neuroscience : the official journal of the Society for Neuroscience 31(38):13625–13634. doi:10.1523/JNEUROSCI.2259-11.2011
Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA et al (2013) Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77(5):955–968. doi:10.1016/j.neuron.2012.12.038
Ramirez S, Liu X, Lin PA, Suh J, Pignatelli M, Redondo RL, Ryan TJ, Tonegawa S (2013) Creating a false memory in the hippocampus. Science 341(6144):387–391. doi:10.1126/science.1239073
Acknowledgments
This work was funded by the National Science Foundation of China (31070954, 81271253, 81471162), the Science and Technology Commission of Shanghai (14JC1402400), and the Key Innovation Project of Shanghai Municipal Education Commission (Grant No.14ZZ090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
The generation of Dcf1 knockout mice. a Verification of F2 deletion at the genomic level. A 700-bp band and lack of a 400-bp band indicate removal of F2 from the genomic DNA. b RT-PCR analysis of Dcf1 expression. The absence of the Dcf1 fragment confirmed gene knockout. c Thy1-EGFP mice were identified by PCR with a transgene (TR) that has a 415-bp band and an internal positive control (IP) that has a 324-bp band. (GIF 19 kb.)
Supplementary Figure 2
Dcf1−/− mice exhibit abnormal spine synapse ultrastructure in area CA3. a Electron micrographs of synapses in WT and Dcf1−/− mice. b Dcf1−/− mice have significantly decreased spine head area. c Frequency histograms of spine head area for WT and Dcf1−/− neurons in area CA3. *P < 0.05, **P < 0.01, one way ANOVA; all data are presented as mean ± SEM; 4 mice per group. (GIF 105 kb.)
Supplementary Figure 3
Dcf1 knockout causes the loss of dendritic spines in layer V/VI at different ages. a Neuronal development at P21, P42, and P90 in layer II/III. Neurons labeled with EGFP appeared at P90 but not at P21 and P42 in this layer. Scale bar: 100 μm. b The density of dendritic spines in layer V/VI of Dcf1−/− mice is decreased in comparison with that of WT mice at P21, P42, and P90. Scale bar: 5 μm. c Statistical comparison of dendritic spine density in layer V/VI of WT and Dcf1−/− mice demonstrates significant differences at P42 and P90. Analysis of dendritic spine density also showed a significant increase in WT mice from P21 to P42 and P90, whereas there was no increase in Dcf1−/− mice across ages. *P < 0.05, **P < 0.01, ***P < 0.001, two way ANOVA; all data are presented as mean ± SEM; 4 or 5 mice per group. (GIF 171 kb.)
Supplementary Figure 4
Dcf1 knockout reduces performance in memory tasks. a In the training trial of the spatial object relocation test, WT and Dcf1−/− mice spent equal time exploring the objects. b In the Morris water maze test, Dcf1−/− mice took significantly longer to locate the hidden platform only in the second trial on the first spatial training day. c-e Swimming path when searching for the hidden platform was much less directed in Dcf1−/− mice c, and the distance they swam on trial 3 d and trial 4 e of the reversal learning task was much longer compared with WT mice. Two tailed t-test, # P < 0.1, *P < 0.05,**P < 0.01,***P < 0.001; all data presented as mean ± SEM. 7–10 mice per group. (GIF 70 kb.)
Supplementary Figure 5
Knocking down the level of Lcn2 in DG area of the hippocampus of KO mice can rescue the learning and memory of KO mice. a The lentivirus of pLKD-Ubc-EGFP-U6-shRNA Lcn2 can reduce the level of Lcn2 contrast the control in the neuro-2A cells. b Pictures showed that the lentivirus was expressed in the DG area of the hippocampus. Two tailed t-test. c Dcf1−/− mice injected with control only took significantly longer to locate the hidden platform contrast WT mice injected with control and KO mice injected with shRNA Lcn2 lentivirus in the second trial on the first spatial training day. Scale bar: 20 μm. # P < 0.1, *P < 0.05,**P < 0.01,***P < 0.001; all data presented as mean ± SEM. 6–9 mice per group. (GIF 99 kb.)
ESM 1
(PDF 47.9 kb.)
Rights and permissions
About this article
Cite this article
Liu, Q., Feng, R., Chen, Y. et al. Dcf1 Triggers Dendritic Spine Formation and Facilitates Memory Acquisition. Mol Neurobiol 55, 763–775 (2018). https://doi.org/10.1007/s12035-016-0349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0349-6