Abstract
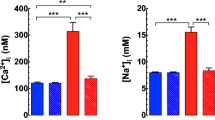
Duchenne muscular dystrophy (DMD) is an inherited X-linked disorder characterized by skeletal muscle wasting, cardiomyopathy, as well as cognitive impairment. Lack of dystrophin in striated muscle produces dyshomeostasis of resting intracellular Ca2+ ([Ca2+]i), Na+ ([Na+]i), and oxidative stress. Here, we test the hypothesis that similar to striated muscle cells, an absence of dystrophin in neurons from mdx mice (a mouse model for DMD) is also associated with dysfunction of [Ca2+]i homeostasis and oxidative stress. [Ca2+]i and [Na+]i in pyramidal cortical and hippocampal neurons from 3 and 6 months mdx mice were elevated compared to WT in an age-dependent manner. Removal of extracellular Ca2+ reduced [Ca2+]i in both WT and mdx neurons, but the decrease was greater and age-dependent in the latter. GsMTx-4 (a blocker of stretch-activated cation channels) significantly decreased [Ca2+]i and [Na+]i in an age-dependent manner in all mdx neurons. Blockade of ryanodine receptors (RyR) or inositol triphosphate receptors (IP3R) reduced [Ca2+]i in mdx. Mdx neurons showed elevated and age-dependent reactive oxygen species (ROS) production and an increase in neuronal damage. In addition, mdx mice showed a spatial learning deficit compared to WT. GsMTx-4 intraperitoneal injection reduced neural [Ca2+]i and improved learning deficit in mdx mice. In summary, mdx neurons show an age-dependent dysregulation in [Ca2+]i and [Na+]i which is mediated by plasmalemmal cation influx and by intracellular Ca2+ release through the RyR and IP3R. Also, mdx neurons have elevated ROS production and more extensive cell damage. Finally, a reduction of [Ca2+]i improved cognitive function in mdx mice.









Similar content being viewed by others
References
Hoffman EP, Hudecki MS, Rosenberg PA, Pollina CM, Kunkel LM (1988) Cell and fiber-type distribution of dystrophin. Neuron 1(5):411–420
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51(6):919–928
Lopez JR, Briceno LE, Sanchez V, Horvart D (1987) Myoplasmic (Ca2+) in Duchenne muscular dystrophy patients. Acta Cient Venez 38(4):503–504
Altamirano F, Lopez JR, Henriquez C, Molinski T, Allen PD, Jaimovich E (2012) Increased resting intracellular calcium modulates NF-kappaB-dependent inducible nitric-oxide synthase gene expression in dystrophic mdx skeletal myotubes. J Biol Chem 287(25):20876–20887. doi:10.1074/jbc.M112.344929
Mijares A, Altamirano F, Kolster J, Adams JA, Lopez JR (2014) Age-dependent changes in diastolic Ca(2+) and Na(+) concentrations in dystrophic cardiomyopathy: role of Ca(2+) entry and IP3. Biochem Biophys Res Commun 452(4):1054–1059. doi:10.1016/j.bbrc.2014.09.045
Blake DJ, Weir A, Newey SE, Davies KE (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82(2):291–329. doi:10.1152/physrev.00028.2001
van Westering TL, Betts CA, Wood MJ (2015) Current understanding of molecular pathology and treatment of cardiomyopathy in duchenne muscular dystrophy. Molecules 20(5):8823–8855. doi:10.3390/molecules20058823
Shirokova N, Niggli E (2013) Cardiac phenotype of Duchenne muscular dystrophy: insights from cellular studies. J Mol Cell Cardiol 58:217–224. doi:10.1016/j.yjmcc.2012.12.009
Nedergaard M, Verkhratsky A (2010) Calcium dyshomeostasis and pathological calcium signalling in neurological diseases. Cell Calcium 47(2):101–102. doi:10.1016/j.ceca.2009.12.011
Blake DJ, Hawkes R, Benson MA, Beesley PW (1999) Different dystrophin-like complexes are expressed in neurons and glia. J Cell Biol 147(3):645–658
Knuesel I, Bornhauser BC, Zuellig RA, Heller F, Schaub MC, Fritschy JM (2000) Differential expression of utrophin and dystrophin in CNS neurons: an in situ hybridization and immunohistochemical study. J Comp Neurol 422(4):594–611
Ahn AH, Kunkel LM (1993) The structural and functional diversity of dystrophin. Nat Genet 3(4):283–291. doi:10.1038/ng0493-283
Leal SL, Yassa MA (2015) Neurocognitive aging and the hippocampus across species. Trends Neurosci 38(12):800–812. doi:10.1016/j.tins.2015.10.003
Anderson JL, Head SI, Rae C, Morley JW (2002) Brain function in Duchenne muscular dystrophy. Brain 125(Pt 1):4–13
Felisari G, Martinelli Boneschi F, Bardoni A, Sironi M, Comi GP, Robotti M, Turconi AC, Lai M et al (2000) Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 55(4):559–564
Nardes F, Araujo AP, Ribeiro MG (2012) Mental retardation in Duchenne muscular dystrophy. J Pediatr 88(1):6–16. doi:10.2223/JPED.2148
Hinton VJ, Fee RJ, Goldstein EM, De Vivo DC (2007) Verbal and memory skills in males with Duchenne muscular dystrophy. Dev Med Child Neurol 49(2):123–128. doi:10.1111/j.1469-8749.2007.00123.x
Miranda R, Sebrie C, Degrouard J, Gillet B, Jaillard D, Laroche S, Vaillend C (2009) Reorganization of inhibitory synapses and increased PSD length of perforated excitatory synapses in hippocampal area CA1 of dystrophin-deficient mdx mice. Cereb Cortex 19(4):876–888. doi:10.1093/cercor/bhn135
Minciacchi D, Del Tongo C, Carretta D, Nosi D, Granato A (2010) Alterations of the cortico-cortical network in sensori-motor areas of dystrophin deficient mice. Neuroscience 166(4):1129–1139. doi:10.1016/j.neuroscience.2010.01.040
Perronnet C, Chagneau C, Le Blanc P, Samson-Desvignes N, Mornet D, Laroche S, De La Porte S, Vaillend C (2012) Upregulation of brain utrophin does not rescue behavioral alterations in dystrophin-deficient mice. Hum Mol Genet 21(10):2263–2276. doi:10.1093/hmg/dds047
Vaillend C, Billard JM, Laroche S (2004) Impaired long-term spatial and recognition memory and enhanced CA1 hippocampal LTP in the dystrophin-deficient Dmd(mdx) mouse. Neurobiol Dis 17(1):10–20. doi:10.1016/j.nbd.2004.05.004
Vaillend C, Rendon A, Misslin R, Ungerer A (1995) Influence of dystrophin-gene mutation on mdx mouse behavior. I. Retention deficits at long delays in spontaneous alternation and bar-pressing tasks. Behav Genet 25(6):569–579
Chaussenot R, Edeline JM, Le Bec B, El Massioui N, Laroche S, Vaillend C (2015) Cognitive dysfunction in the dystrophin-deficient mouse model of Duchenne muscular dystrophy: a reappraisal from sensory to executive processes. Neurobiol Learn Mem 124:111–122. doi:10.1016/j.nlm.2015.07.006
Brini M, Cali T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71(15):2787–2814. doi:10.1007/s00018-013-1550-7
Lopez JR, Lyckman A, Oddo S, Laferla FM, Querfurth HW, Shtifman A (2008) Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J Neurochem 105(1):262–271. doi:10.1111/j.1471-4159.2007.05135.x
Turner PR, Westwood T, Regen CM, Steinhardt RA (1988) Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335(6192):735–738. doi:10.1038/335735a0
Altamirano F, Perez CF, Liu M, Widrick J, Barton ER, Allen PD, Adams JA, Lopez JR (2014) Whole body periodic acceleration is an effective therapy to ameliorate muscular dystrophy in mdx mice. PLoS One 9(9):e106590. doi:10.1371/journal.pone.0106590
Altamirano F, Valladares D, Henriquez-Olguin C, Casas M, Lopez JR, Allen PD, Jaimovich E (2013) Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice. PLoS One 8(12):e81222. doi:10.1371/journal.pone.0081222
Hopf FW, Steinhardt RA (1992) Regulation of intracellular free calcium in normal and dystrophic mouse cerebellar neurons. Brain Res 578(1–2):49–54
Haws CM, Lansman JB (1991) Calcium-permeable ion channels in cerebellar neurons from mdx mice. Proc Biol Sci 244(1311):185–189. doi:10.1098/rspb.1991.0068
Carretta D, Santarelli M, Vanni D, Ciabatti S, Sbriccoli A, Pinto F, Minciacchi D (2003) Cortical and brainstem neurons containing calcium-binding proteins in a murine model of Duchenne’s muscular dystrophy: selective changes in the sensorimotor cortex. J Comp Neurol 456(1):48–59. doi:10.1002/cne.10506
Spencer MJ, Tidball JG (1992) Calpain concentration is elevated although net calcium-dependent proteolysis is suppressed in dystrophin-deficient muscle. Exp Cell Res 203(1):107–114
Tuckett E, Gosetti T, Hayes A, Rybalka E, Verghese E (2015) Increased calcium in neurons in the cerebral cortex and cerebellum is not associated with cell loss in the mdx mouse model of Duchenne muscular dystrophy. Neuroreport 26(13):785–790. doi:10.1097/WNR.0000000000000425
Floyd RA (1999) Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med 222(3):236–245
Droge W (2003) Oxidative stress and aging. Adv Exp Med Biol 543:191–200
Terrill JR, Radley-Crabb HG, Iwasaki T, Lemckert FA, Arthur PG, Grounds MD (2013) Oxidative stress and pathology in muscular dystrophies: focus on protein thiol oxidation and dysferlinopathies. FEBS J 280(17):4149–4164. doi:10.1111/febs.12142
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40. doi:10.1016/j.cbi.2005.12.009
Durany N, Munch G, Michel T, Riederer P (1999) Investigations on oxidative stress and therapeutical implications in dementia. Eur Arch Psychiatry Clin Neurosci 249(Suppl 3):68–73
Abraham S, Soundararajan CC, Vivekanandhan S, Behari M (2005) Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J Med Res 121(2):111–115
Comim CM, Cassol-Jr OJ, Constantino LC, Constantino LS, Petronilho F, Tuon L, Vainzof M, Dal-Pizzol F et al (2009) Oxidative variables and antioxidant enzymes activities in the mdx mouse brain. Neurochem Int 55(8):802–805. doi:10.1016/j.neuint.2009.08.003
Zundorf G, Reiser G (2011) Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal 14(7):1275–1288. doi:10.1089/ars.2010.3359
Xu X, Zhan M, Duan W, Prabhu V, Brenneman R, Wood W, Firman J, Li H et al (2007) Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol 8(11):R234. doi:10.1186/gb-2007-8-11-r234
Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429(6994):883–891. doi:10.1038/nature02661
Viel JJ, McManus DQ, Cady C, Evans MS, Brewer GJ (2001) Temperature and time interval for culture of postmortem neurons from adult rat cortex. J Neurosci Res 64(4):311–321
Shen X, Ma L, Dong W, Wu Q, Gao Y, Luo C, Zhang M, Chen X et al (2016) Autophagy regulates intracerebral hemorrhage induced neural damage via apoptosis and NF-kappaB pathway. Neurochem Int 96:100–112. doi:10.1016/j.neuint.2016.03.004
Pal S, Hartnett KA, Nerbonne JM, Levitan ES, Aizenman E (2003) Mediation of neuronal apoptosis by Kv2.1-encoded potassium channels. The Journal of neuroscience : the official journal of the Society for Neuroscience 23(12):4798–4802
Eltit JM, Ding X, Pessah IN, Allen PD, Lopez JR (2013) Nonspecific sarcolemmal cation channels are critical for the pathogenesis of malignant hyperthermia. FASEB J 27(3):991–1000. doi:10.1096/fj.12-218354
Lopez JR, Linares N, Pessah IN, Allen PD (2005) Enhanced response to caffeine and 4-chloro-m-cresol in malignant hyperthermia-susceptible muscle is related in part to chronically elevated resting [Ca2+]i. Am J Physiol Cell Physiol 288(3):C606–C612. doi:10.1152/ajpcell.00297.2004
Taylor PJ, Betts GA, Maroulis S, Gilissen C, Pedersen RL, Mowat DR, Johnston HM, Buckley MF (2010) Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS One 5(1):e8803. doi:10.1371/journal.pone.0008803
Wu H, Jin Y, Arias J, Bassuk J, Uryash A, Kurlansky P, Webster K, Adams JA (2009) In vivo upregulation of nitric oxide synthases in healthy rats. Nitric oxide: biology and chemistry / official journal of the Nitric Oxide Society 21(1):63–68. doi:10.1016/j.niox.2009.05.004
Handattu SP, Garber DW, Monroe CE, van Groen T, Kadish I, Nayyar G, Cao D, Palgunachari MN et al (2009) Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer’s disease. Neurobiol Dis 34(3):525–534. doi:10.1016/j.nbd.2009.03.007
Faes C, Aerts M, Geys H, De Schaepdrijver L (2010) Modeling spatial learning in rats based on Morris water maze experiments. Pharm Stat 9(1):10–20. doi:10.1002/pst.361
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60
Sesay AK, Errington ML, Levita L, Bliss TV (1996) Spatial learning and hippocampal long-term potentiation are not impaired in mdx mice. Neurosci Lett 211(3):207–210
Block F (1999) Global ischemia and behavioural deficits. Prog Neurobiol 58(3):279–295
Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG (2005) Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol 562(Pt 2):367–380. doi:10.1113/jphysiol.2004.075275
Ward ML, Williams IA, Chu Y, Cooper PJ, Ju YK, Allen DG (2008) Stretch-activated channels in the heart: contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Biol 97(2–3):232–249. doi:10.1016/j.pbiomolbio.2008.02.009
Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F (2000) Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol 115(5):583–598
Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN (1997) Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19(3):723–733
Ohashi R, Sakata S, Naito A, Hirashima N, Tanaka M (2014) Dendritic differentiation of cerebellar Purkinje cells is promoted by ryanodine receptors expressed by Purkinje and granule cells. Dev Neurobiol 74(4):467–480. doi:10.1002/dneu.22139
Lidov HG (1996) Dystrophin in the nervous system. Brain Pathol 6(1):63–77
Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM et al (2007) Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 27(12):3098–3110. doi:10.1523/JNEUROSCI.4163-06.2007
Gleichmann M, Mattson MP (2011) Neuronal calcium homeostasis and dysregulation. Antioxid Redox Signal 14(7):1261–1273. doi:10.1089/ars.2010.3386
Raza M, Deshpande LS, Blair RE, Carter DS, Sombati S, DeLorenzo RJ (2007) Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons. Neurosci Lett 418(1):77–81. doi:10.1016/j.neulet.2007.03.005
Bezprozvanny I, Mattson MP (2008) Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci 31(9):454–463. doi:10.1016/j.tins.2008.06.005
Alvarez-Leefmans FJ, Rink TJ, Tsien RY (1981) Free calcium ions in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol 315:531–548
Tsien RY, Rink TJ (1981) Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods 4(1):73–86
Mehler MF, Haas KZ, Kessler JA, Stanton PK (1992) Enhanced sensitivity of hippocampal pyramidal neurons from mdx mice to hypoxia-induced loss of synaptic transmission. Proc Natl Acad Sci U S A 89(6):2461–2465
Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F (2007) Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49(2):249–270. doi:10.1016/j.toxicon.2006.09.030
Bode F, Sachs F, Franz MR (2001) Tarantula peptide inhibits atrial fibrillation. Nature 409(6816):35–36. doi:10.1038/35051165
Firth AL, Remillard CV, Yuan JX (2007) TRP channels in hypertension. Biochim Biophys Acta 1772(8):895–906. doi:10.1016/j.bbadis.2007.02.009
Lanner JT (2012) Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol 740:217–234. doi:10.1007/978-94-007-2888-2_9
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1(1):11–21. doi:10.1038/35036035
Verkhratsky A (2005) Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85(1):201–279. doi:10.1152/physrev.00004.2004
Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S et al (2006) Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2(11):596–607. doi:10.1038/nchembio821
Tracey I, Scott RB, Thompson CH, Dunn JF, Barnes PR, Styles P, Kemp GJ, Rae CD et al (1995) Brain abnormalities in Duchenne muscular dystrophy: phosphorus-31 magnetic resonance spectroscopy and neuropsychological study. Lancet 345(8960):1260–1264
Kemp GJ, Taylor DJ, Dunn JF, Frostick SP, Radda GK (1993) Cellular energetics of dystrophic muscle. J Neurol Sci 116(2):201–206
Pulido SM, Passaquin AC, Leijendekker WJ, Challet C, Wallimann T, Ruegg UT (1998) Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Lett 439(3):357–362
Griffin JL, Williams HJ, Sang E, Clarke K, Rae C, Nicholson JK (2001) Metabolic profiling of genetic disorders: a multitissue (1)H nuclear magnetic resonance spectroscopic and pattern recognition study into dystrophic tissue. Anal Biochem 293(1):16–21. doi:10.1006/abio.2001.5096
Rae C, Griffin JL, Blair DH, Bothwell JH, Bubb WA, Maitland A, Head S (2002) Abnormalities in brain biochemistry associated with lack of dystrophin: studies of the mdx mouse. Neuromuscul Disord 12(2):121–129
Wang H, Joseph JA (2000) Mechanisms of hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic Biol Med 28(8):1222–1231
Oliveira AM, Bading H, Mauceri D (2014) Dysfunction of neuronal calcium signaling in aging and disease. Cell Tissue Res 357(2):381–383. doi:10.1007/s00441-014-1954-1
Paula-Lima AC, Adasme T, Hidalgo C (2014) Contribution of Ca2+ release channels to hippocampal synaptic plasticity and spatial memory: potential redox modulation. Antioxid Redox Signal 21(6):892–914. doi:10.1089/ars.2013.5796
Turk R, Sterrenburg E, de Meijer EJ, van Ommen GJ, den Dunnen JT, t Hoen PA (2005) Muscle regeneration in dystrophin-deficient mdx mice studied by gene expression profiling. BMC Genomics 6:98. doi:10.1186/1471-2164-6-98
Lidov HG, Byers TJ, Kunkel LM (1993) The distribution of dystrophin in the murine central nervous system: an immunocytochemical study. Neuroscience 54(1):167–187
Gorecki D, Geng Y, Thomas K, Hunt SP, Barnard EA, Barnard PJ (1991) Expression of the dystrophin gene in mouse and rat brain. Neuroreport 2(12):773–776
Muntoni F, Mateddu A, Serra G (1991) Passive avoidance behaviour deficit in the mdx mouse. Neuromuscul Disord 1(2):121–123
Vaillend C, Billard JM, Claudepierre T, Rendon A, Dutar P, Ungerer A (1998) Spatial discrimination learning and CA1 hippocampal synaptic plasticity in mdx and mdx3cv mice lacking dystrophin gene products. Neuroscience 86(1):53–66
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36(1):60–90
Brandeis R, Brandys Y, Yehuda S (1989) The use of the Morris water maze in the study of memory and learning. Int J Neurosci 48(1–2):29–69
Begley DJ (2004) ABC transporters and the blood-brain barrier. Curr Pharm Des 10(12):1295–1312
Tonkikh A, Janus C, El-Beheiry H, Pennefather PS, Samoilova M, McDonald P, Ouanounou A, Carlen PL (2006) Calcium chelation improves spatial learning and synaptic plasticity in aged rats. Exp Neurol 197(2):291–300. doi:10.1016/j.expneurol.2005.06.014
Lu CB, Hamilton JB, Powell AD, Toescu EC, Vreugdenhil M (2011) Effect of ageing on CA3 interneuron sAHP and gamma oscillations is activity-dependent. Neurobiol Aging 32(5):956–965. doi:10.1016/j.neurobiolaging.2009.05.006
Tsukuda K, Mogi M, Li JM, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M et al (2008) Diabetes-associated cognitive impairment is improved by a calcium channel blocker, nifedipine. Hypertension 51(2):528–533. doi:10.1161/HYPERTENSIONAHA.107.101634
Mehler MF (2000) Brain dystrophin, neurogenetics and mental retardation. Brain Res Brain Res Rev 32(1):277–307
Acknowledgements
Florida Heart Research Institute (JAA), IDAs University of California 3-V440LP2 (JRL) supported the research reported in this publication. We would like to thank Dr. Ray Zhang for his help in statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Supplementary Fig. 1
Expression of Dp71 and utrophin. a Representative Western blots of Dp71 and utrophin (UTR) and respective protein loading control Tubulin, in 3- and 6-month-old WT and mdx neurons. Expression of Dp71 and UTR was reduced in an age-dependent manner in cortical neurons of mdx mice. b Protein expression of Dp71 was significantly reduced in 3- and 6-month mdx neurons compared to age-matched WT (p < 0.001). c Protein expression of utrophin was also significantly reduced in both 3- and 6-month mdx neurons compared to age-matched WT (p < 0.001). (GIF 85 kb)
Supplementary Fig. 2
Protein level of α- and β-dystroglycan and sarcoglycan. a. Representative Western blots of α (α-DG) and β (β-DG) dystroglycan and α (α-SG) and β (β-SG) sarcoglycan and respective protein loading control Tubulin in 3- and 6-month-old WT and mdx neurons. Expression of α- and β-dystroglycan was reduced in an age-dependent manner (p < 0.001) in mdx cortical neurons; however, the decrease was most marked for β- than α-dystroglycan. Expression of α- and β-sarcoglycan was also significantly different between WT and mdx at either 3 or 6 months. b Protein expression of α-DG was significantly reduced in 6-month mdx neurons compared to age-matched WT (p < 0.001). c Protein expression of β-DG was also significantly reduced in both 3- and 6-month mdx neurons compared to age-matched WT (p < 0.001). d α-SG was significantly reduced in mdx cortical neurons at both 3 and 6 months compared to WT (p < 0.01). e β-SG was also significantly reduced in mdx cortical neurons at both 3 and 6 months compared to WT (p < 0.001) (GIF 82 kb)
Supplementary Fig. 3
Transient receptor potential cation channel (TRPC)-1, TRPC-4, and TRPC-6 protein levels. a Representative Western blots of transient receptor potential cation channels (TRPC-1, TRPC-4, and TRPC-6) and respective protein loading control Tubulin, in 3- and 6-month-old WT and mdx neurons. b TRPC-1 expression was unchanged at 3 months between WT and mdx. In contrast, TRPC-1 was significantly overexpressed in 6-month-old mdx neurons compared to WT (p < 0.001). c TRPC-4 was significantly overexpressed in both 3- and 6-month mdx neurons compared to WT age-matched controls (p < 0.01). d TRPC-6 expression was unchanged at 3 months between WT and mdx but significantly overexpressed in 6-month-old mdx neurons compared to age-matched WT (p < 0.001). (GIF 102 kb)
Rights and permissions
About this article
Cite this article
Lopez, J.R., Kolster, J., Uryash, A. et al. Dysregulation of Intracellular Ca2+ in Dystrophic Cortical and Hippocampal Neurons. Mol Neurobiol 55, 603–618 (2018). https://doi.org/10.1007/s12035-016-0311-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0311-7