Abstract
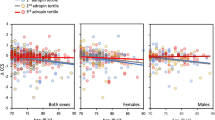
Decreased β-amyloid (Aβ) clearance from the brain has been suggested to contribute to cerebral Aβ accumulation in Alzheimer’s disease. Based on the idea of a dynamic Aβ equilibrium in different body compartments, plasma Aβ levels have been investigated as biomarker candidates for preclinical Alzheimer’s pathology, yet with inconsistent results. Since the kidneys are involved in Aβ elimination from the blood, we evaluated how chronic kidney disease (CKD) affects the association between plasma Aβ and cognitive deficits and cognitive decline. In 28 CKD patients, stages 3–5D, and 26 control subjects with comparable vascular risk profile from the New Tools for the Prevention of Cardiovascular Disease in Chronic Kidney Disease (NTCVD) cohort, plasma total Aβ was determined with a highly sensitive electrochemiluminescence immunoassay. Cognition was evaluated using a comprehensive battery of ten neuropsychological tests at baseline and 2-year follow-up. Subjects with high plasma Aβ level (above median) demonstrated a significantly worse baseline cognitive performance than subjects exhibiting low Aβ level (summary score of global cognitive performance at baseline z = −0.46 ± 0.76 vs z = −0.08 ± 0.57, p = 0.045). Cognitive performance moderately decreased over the 2-year follow-up in subjects with high plasma Aβ level (Δz = −0.13 ± 0.51), but increased in subjects with low plasma Aβ level (Δz = 0.16 ± 0.41, p = 0.023). In linear regression analyses, baseline plasma Aβ was significantly associated with cognitive decline both in unadjusted analyses (β = −0.28, 95% CI = −0.55 to −0.01) and analyses adjusted for age (β = −0.27, 95% CI = −0.54 to −0.01). Our results suggest the utility of plasma Aβ level in predicting cognitive decline in patients suffering from CKD.


Similar content being viewed by others
References
Abdullah L, Paris D, Luis C, Quadros A, Parrish J, Valdes L, Keegan AP, Mathura V et al (2007) The influence of diagnosis, intra- and inter-person variability on serum and plasma Abeta levels. Neurosci Lett 428(2–3):53–58. doi:10.1016/j.neulet.2007.09.058
Arvanitakis Z, Lucas JA, Younkin LH, Younkin SG, Graff-Radford NR (2002) Serum creatinine levels correlate with plasma amyloid Beta protein. Alzheimer Dis Assoc Disord 16(3):187–190
Aschenbrenner S, Tucha O, Lange KW (2000) Regensburger Wortflüssigkeits-Test (RWT). Hogrefe, Göttingen
Bäumler G (1985) Farb-Wort-Interferenztest (FWIT) nach J.R. Stroop. Hogrefe, Göttingen
Cammarata S, Borghi R, Giliberto L, Pardini M, Pollero V, Novello C, Fornaro M, Vitali A et al (2009) Amyloid-beta42 plasma levels are elevated in amnestic mild cognitive impairment. J Alzheimer’s Dis: JAD 18(2):267–271. doi:10.3233/jad-2009-1144
Chiu MJ, Yang SY, Horng HE, Yang CC, Chen TF, Chieh JJ, Chen HH, Chen TC et al (2013) Combined plasma biomarkers for diagnosing mild cognition impairment and Alzheimer’s disease. ACS Chem Neurosci 4(12):1530–1536. doi:10.1021/cn400129p
Cosentino SA, Stern Y, Sokolov E, Scarmeas N, Manly JJ, Tang MX, Schupf N, Mayeux RP (2010) Plasma ss-amyloid and cognitive decline. Arch Neurol 67(12):1485–1490. doi:10.1001/archneurol.2010.189
Fullwood NJ, Hayashi Y, Allsop D (2006) Plasma amyloid-beta concentrations in Alzheimer’s disease: an alternative hypothesis. Lancet Neurol 5(12):1000–1001. doi:10.1016/s1474-4422(06)70611-1author reply 1002-1003
Gronewold J, Klafki HW, Baldelli E, Kaltwasser B, Seidel UK, Todica O, Volsek M, Haussmann U et al (2015) Factors responsible for plasma beta-amyloid accumulation in chronic kidney disease. Mol Neurobiol. doi:10.1007/s12035-015-9218-y
Hansson O, Stomrud E, Vanmechelen E, Ostling S, Gustafson DR, Zetterberg H, Blennow K, Skoog I (2012) Evaluation of plasma Abeta as predictor of Alzheimer’s disease in older individuals without dementia: a population-based study. J Alzheimer’s Dis: JAD 28(1):231–238. doi:10.3233/jad-2011-111418
Härting C, Markowitsch HJ, Neufeld U, Calabrese P, Deisinger K, Kessler J (2000) Wechsler Memory Scale—revised edition, German edn. Huber, Bern
Herrmann-Lingen C, Buss U, Snaith RP (2005) Hospital Anxiety and Depression Scale - Deutsche Version (HADS-D). Hans Huber, Bern
Jack CR Jr, Holtzman DM (2013) Biomarker modeling of Alzheimer’s disease. Neuron 80(6):1347–1358. doi:10.1016/j.neuron.2013.12.003
Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F (2012) Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 69(7):824–831. doi:10.1001/archneurol.2011.1841
Kuo YM, Kokjohn TA, Kalback W, Luehrs D, Galasko DR, Chevallier N, Koo EH, Emmerling MR et al (2000) Amyloid-beta peptides interact with plasma proteins and erythrocytes: implications for their quantitation in plasma. Biochem Biophys Res Commun 268(3):750–756. doi:10.1006/bbrc.2000.2222
Liu YH, Xiang Y, Wang YR, Jiao SS, Wang QH, Bu XL, Zhu C, Yao XQ et al (2014) Association between serum amyloid-beta and renal functions: implications for roles of kidney in amyloid-beta clearance. Mol Neurobiol. doi:10.1007/s12035-014-8854-y
Liu YH, Wang YR, Xiang Y, Zhou HD, Giunta B, Manucat-Tan NB, Tan J, Zhou XF et al (2015) Clearance of amyloid-beta in Alzheimer’s disease: shifting the action site from center to periphery. Mol Neurobiol 51(1):1–7. doi:10.1007/s12035-014-8694-9
Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA, Chang YF, Tracy R et al (2008) Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology 70(19):1664–1671. doi:10.1212/01.wnl.0000306696.82017.66
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science (New York, NY) 330(6012):1774. doi:10.1126/science.1197623
Nettiksimmons J, Ayonayon H, Harris T, Phillips C, Rosano C, Satterfield S, Yaffe K (2015) Development and validation of risk index for cognitive decline using blood-derived markers. Neurology 84(7):696–702. doi:10.1212/wnl.0000000000001263
Okereke OI, Xia W, Selkoe DJ, Grodstein F (2009) Ten-year change in plasma amyloid beta levels and late-life cognitive decline. Arch Neurol 66(10):1247–1253. doi:10.1001/archneurol.2009.207
Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C et al (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. doi:10.1016/s1474-4422(16)00070-3
Panza F, Solfrizzi V, Imbimbo BP, Logroscino G (2014) Amyloid-directed monoclonal antibodies for the treatment of Alzheimer’s disease: the point of no return? Expert Opin Biol Ther 14(10):1465–1476. doi:10.1517/14712598.2014.935332
Pilotto A, Panza F, Sancarlo D, Paroni G, Maggi S, Ferrucci L (2012) Usefulness of the multidimensional prognostic index (MPI) in the management of older patients with chronic kidney disease. J Nephrol 25(Suppl 19):S79–S84. doi:10.5301/jn.5000162
Poljak A, Crawford JD, Smythe GA, Brodaty H, Slavin MJ, Kochan NA, Trollor JN, Wen W, et al (2016) The relationship between plasma Abeta levels, cognitive function and brain volumetrics: Sydney Memory and Ageing Study. Curr Alzheimer Res 13(3):243–255.
Pomara N, Willoughby LM, Sidtis JJ, Mehta PD (2005) Selective reductions in plasma Abeta 1-42 in healthy elderly subjects during longitudinal follow-up: a preliminary report. Am J Geriatr Psychiatr: Off J Am Assoc Geriatr Psychiatr 13(10):914–917. doi:10.1176/appi.ajgp.13.10.914
Reitan RM (1992) Trail making test. Reitan Neuropsychology Laboratory, Tucson, AZ
Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC et al (2009) Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimer’s & Dementia : J Alzheimer’s Assoc 5(1):18–29. doi:10.1016/j.jalz.2008.10.004
Russ M (2002) Rey complex figure test. Anleitung für die Anwendung im Frankfurter Neuropsychologischen Testprofil (FNTP). http://www.fntp.de/mediapool/80/807841/data/CFT_Scoring.pdf. Accessed 5 Sept 2012
Salem S, Bruck H, Bahlmann FH, Peter M, Passlick-Deetjen J, Kretschmer A, Steppan S, Volsek M et al (2012) Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am J Nephrol 35(1):31–39. doi:10.1159/000334742
Seidel UK, Gronewold J, Volsek M, Todica O, Kribben A, Bruck H, Hermann DM (2014) The prevalence, severity, and association with HbA1c and fibrinogen of cognitive impairment in chronic kidney disease. Kidney Int 85(3):693–702. doi:10.1038/ki.2013.366
Seppala TT, Herukka SK, Hanninen T, Tervo S, Hallikainen M, Soininen H, Pirttila T (2010) Plasma Abeta42 and Abeta40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry 81(10):1123–1127. doi:10.1136/jnnp.2010.205757
Song F, Poljak A, Valenzuela M, Mayeux R, Smythe GA, Sachdev PS (2011) Meta-analysis of plasma amyloid-beta levels in Alzheimer’s disease. J Alzheimer’s Dis: JAD 26(2):365–375. doi:10.3233/jad-2011-101977
Takata M, Nakashima M, Takehara T, Baba H, Machida K, Akitake Y, Ono K, Hosokawa M et al (2008) Detection of amyloid beta protein in the urine of Alzheimer’s disease patients and healthy individuals. Neurosci Lett 435(2):126–130. doi:10.1016/j.neulet.2008.02.019
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H et al (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11(8):457–470. doi:10.1038/nrneurol.2015.119
Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, Younkin LH, Kuller L et al (2011) Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 305(3):261–266. doi:10.1001/jama.2010.1995
Acknowledgments
We thank Gisela Behrendt for the administrative and technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by the Federal Ministry of Education and Research (BMBF/NGFN 01GR0808), which was not involved in the study design, in the collection, analysis, and interpretation of data; in writing the report; and in the decision to submit the article for publication.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Gronewold, J., Todica, O., Klafki, HW. et al. Association of Plasma β-Amyloid with Cognitive Performance and Decline in Chronic Kidney Disease. Mol Neurobiol 54, 7194–7203 (2017). https://doi.org/10.1007/s12035-016-0243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0243-2