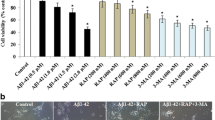
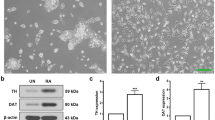
Abstract
Autophagy is a catabolic process involved in the continuous removal of toxic protein aggregates and cellular organelles to maintain the homeostasis and functional integrity of cells. The mechanistic understanding of autophagy mediated neuroprotection during the development of neurodegenerative disorders remains elusive. Here, we investigated the potential role of rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB pathway(s) in the neuroprotection of amyloid-beta (Aβ1-42)-insulted hippocampal neurons in rat model of Alzheimer’s disease (AD) like phenotypes. A single intra-hippocampal injection of Aβ1-42 impaired redox balance and markedly induced synaptic dysfunction, neurotransmission dysfunction, and cognitive deficit, and suppressed pro-survival signaling in the adult rats. Rapamycin administration caused a significant reduction of mTOR complex 1 phosphorylation at Ser2481 and a significant increase in levels of autophagy markers such as microtubule-associated protein-1 light chain-3 (LC3), beclin-1, sequestosome-1/p62, unc-51-like kinase 1 (ULK1). In addition, rapamycin induced the activation of autophagy that further activated p-PI3K, p-Akt1 (Ser473), and p-CREB (Ser183) expression in Aβ1-42-treated rats. The activated autophagy markedly reversed Aβ1-42-induced impaired redox homeostasis by decreasing the levels of prooxidants—ROS generation, intracellular Ca2+ flux and LPO, and increasing the levels of antioxidants—SOD, catalase, and GSH. The activated autophagy also provided significant neuroprotection against Aβ1-42-induced synaptic dysfunction by increasing the expression of synapsin-I, synaptophysin, and PSD95; and neurotransmission dysfunction by increasing the levels of CHRM2, DAD2 receptor, NMDA receptor, and AMPA receptor; and ultimately improved cognitive ability in rats. Wortmannin administration significantly reduced the expression of autophagy markers, p-PI3K, p-Akt1, and p-CREB, as well as the autophagy mediated neuroprotective effect. Our study demonstrate that autophagy can be an integrated part of pro-survival (PI3K/Akt1/mTOR/CREB) signaling and autophagic activation restores the oxidative defense mechanism(s), neurodegenerative damages, and maintains the integrity of synapse and neurotransmission in rat model of AD.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Sanchez PE, Zhu L, Verret L et al (2012) Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc Natl Acad Sci 109:E2895–E2903
Martínez E, Navarro A, Ordóñez C et al (2013) Oxidative stress induces apolipoprotein D overexpression in hippocampus during aging and Alzheimer’s disease. J Alzheimers Dis JAD 36:129–144
Melov S, Adlard PA, Morten K et al (2007) Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS One 2:e536
Chen Y, Wei G, Nie H et al (2014) β-Asarone prevents autophagy and synaptic loss by reducing ROCK expression in a senescence-accelerated prone 8 mice. Brain Res 1552:41–54
Trepanier CH, Jackson MF, MacDonald JF (2012) Regulation of NMDA receptors by the tyrosine kinase Fyn: regulation of NMDA receptors. FEBS J 279:12–19
Palop JJ, Chin J, Roberson ED et al (2007) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55:697–711
Alberdi E, Sánchez-Gómez MV, Cavaliere F et al (2010) Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47:264–272
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741
Cuervo AM, Bergamini E, Brunk UT et al (2005) Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1:131–140
Ling D, Salvaterra PM (2009) A central role for autophagy in Alzheimer-type neurodegeneration. Autophagy 5:738–740
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13:805–811
Nixon RA, Wegiel J, Kumar A et al (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol 64:113–122
Yu WH, Cuervo AM, Kumar A et al (2005) Macroautophagy—a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 171:87–98
Zhang J, Zhang Y, Li J et al (2012) Autophagosomes accumulation is associated with β-amyloid deposits and secondary damage in the thalamus after focal cortical infarction in hypertensive rats: autophagy and β-amyloid after cortical infarction. J Neurochem 120:564–573
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Sarkar S (2013) Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans 41:1103–1130
Kim DH, Sarbassov DD, Ali SM et al (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175
Schmelzle T, Hall MN (2000) TOR, a central controller of cell growth. Cell 103:253–262
Wouters BG, Koritzinsky M (2008) Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 8:851–864
Berger Z, Ravikumar B, Menzies FM et al (2006) Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 15:433–442
Nixon RA (2006) Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci 29:528–535
Bagheri M, Joghataei MT, Mohseni S, Roghani M (2011) Genistein ameliorates learning and memory deficits in amyloid β(1–40) rat model of Alzheimer’s disease. Neurobiol Learn Mem 95:270–276
Chauhan A, Sharma U, Jagannathan NR et al (2011) Rapamycin protects against middle cerebral artery occlusion induced focal cerebral ischemia in rats. Behav Brain Res 225:603–609
Xu JT, Tu HY, Xin WJ et al (2007) Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 206:269–279
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27:612–616
Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330:613–622
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Hong IS, Lee H-Y, Kim HP (2014) Anti-oxidative effects of rooibos tea (Aspalathus linearis) on immobilization-induced oxidative stress in rat brain. PLoS One 9:e87061
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Kashyap MP, Singh AK, Yadav DK et al (2015) 4-Hydroxy-trans-2-nonenal (4-HNE) induces neuronal SH-SY5Y cell death via hampering ATP binding at kinase domain of Akt1. Arch Toxicol 89:243–258
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Seth PK, Alleva FR, Balazs T (1982) Alteration of high-affinity binding sites of neurotransmitter receptors in rats after neonatal exposure to streptomycin. Neurotoxicology 3:13–19
Moreira EG, Vassilieff I, Vassilieff VS (2001) Developmental lead exposure: behavioral alterations in the short and long term. Neurotoxicol Teratol 23:489–495
Ali T, Yoon GH, Shah SA et al (2015) Osmotin attenuates amyloid beta-induced memory impairment, tau phosphorylation and neurodegeneration in the mouse hippocampus. Sci Rep 5:11708
Brouillette J, Caillierez R, Zommer N et al (2012) Neurotoxicity and memory deficits induced by soluble low-molecular-weight amyloid-1-42 oligomers are revealed in vivo by using a novel animal model. J Neurosci 32:7852–7861
Flynn JM, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12
Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294
Yao J, Du H, Yan S et al (2011) Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces a accumulation and improves mitochondrial function in a mouse model of Alzheimer’s disease. J Neurosci 31:2313–2320
Sheehan JP, Swerdlow RH, Miller SW et al (1997) Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. J Neurosci 17:4612–4622
Damme M, Suntio T, Saftig P, Eskelinen E-L (2015) Autophagy in neuronal cells: general principles and physiological and pathological functions. Acta Neuropathol (Berl) 129:337–362
Li L, Zhang X, Le W (2010) Autophagy dysfunction in Alzheimer’s disease. Neurodegener Dis 7:265–271
Efeyan A, Comb WC, Sabatini DM (2015) Nutrient-sensing mechanisms and pathways. Nature 517:302–310
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101
Abada A, Elazar Z (2014) Getting ready for building: signaling and autophagosome biogenesis. EMBO Rep 15:839–852
Zou J, Yue F, Jiang X et al (2013) Mitochondrion-associated protein LRPPRC suppresses the initiation of basal levels of autophagy via enhancing Bcl-2 stability. Biochem J 454:447–457
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Jaeger PA, Pickford F, Sun CH et al (2010) Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One 5:e11102
Gibson SB (2013) Investigating the Role of Reactive Oxygen Species in Regulating Autophagy. In: Methods Enzymol. Elsevier, pp 217–235
Salminen A, Kaarniranta K, Haapasalo A et al (2012) Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer’s disease. Prog Neurobiol 96:87–95
Kitagishi Y, Nakanishi A, Ogura Y, Matsuda S (2014) Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers Res Ther 6:35
Tong L, Thornton PL, Balazs R, Cotman CW (2001) Beta-amyloid-(1-42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival is not compromised. J Biol Chem 276:17301–17306
Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu Rev Neurosci 21:127–148
Pugazhenthi S, Wang M, Pham S et al (2011) Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener 6:60
O’Reilly KE, Rojo F, She QB et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Palop JJ, Mucke L (2010) Amyloid-β–induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 13:812–818
Shen W, Ganetzky B (2009) Autophagy promotes synapse development in Drosophila. J Cell Biol 187:71–79
Yang DS, Stavrides P, Saito M et al (2014) Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits. Brain 137:3300–3318
Collingridge GL, Isaac JTR, Wang YT (2004) Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 5:952–962
Kar S, Slowikowski SPM, Westaway D, Mount HTJ (2004) Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci JPN 29:427–441
Attems J, Quass M, Jellinger KA (2007) Tau and alpha-synuclein brainstem pathology in Alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol (Berl) 113:53–62
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Chang KT, Berg DK (2001) Voltage-gated channels block nicotinic regulation of CREB phosphorylation and gene expression in neurons. Neuron 32:855–865
Nair VD, Sealfon SC (2003) Agonist-specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J Biol Chem 278:47053–47061
Janssen WGM, Vissavajjhala P, Andrews G et al (2005) Cellular and synaptic distribution of NR2A and NR2B in macaque monkey and rat hippocampus as visualized with subunit-specific monoclonal antibodies. Exp Neurol 191(Suppl 1):S28–S44
Mishizen-Eberz AJ, Rissman RA, Carter TL et al (2004) Biochemical and molecular studies of NMDA receptor subunits NR1/2 A/2B in hippocampal subregions throughout progression of Alzheimer’s disease pathology. Neurobiol Dis 15:80–92
Paula-Lima AC, Brito-Moreira J, Ferreira ST (2013) Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer’s disease. J Neurochem 126:191–202
Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11:682–696
Wang Q, Zengin A, Ying W et al (2008) Chronic treatment with simvastatin upregulates muscarinic M1/4 receptor binding in the rat brain. Neuroscience 154:1100–1106
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66:137–147
Matsuda S, Miura E, Matsuda K et al (2008) Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron 57:730–745
Morrison JH, Baxter MG (2014) Synaptic health. JAMA Psychiatry 71:835
Spilman P, Podlutskaya N, Hart MJ et al (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLoS One 5:e9979
Acknowledgments
Dr. D. S. Kothari Post-Doctoral Fellowship scheme of the University Grant Commission, New Delhi, India, is acknowledged for providing financial support (F.4-2/2006(BSR)/BL/14-15/0326) and fellowship to Dr. A. K. Singh. The Department of Biochemistry, University of Allahabad is a recipient of the FIST grant from the DST SERB, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Singh, A.K., Kashyap, M.P., Tripathi, V.K. et al. Neuroprotection Through Rapamycin-Induced Activation of Autophagy and PI3K/Akt1/mTOR/CREB Signaling Against Amyloid-β-Induced Oxidative Stress, Synaptic/Neurotransmission Dysfunction, and Neurodegeneration in Adult Rats. Mol Neurobiol 54, 5815–5828 (2017). https://doi.org/10.1007/s12035-016-0129-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0129-3