Abstract
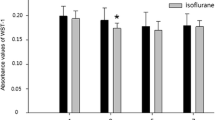
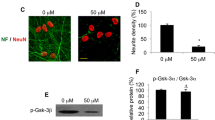
Ketamine is used as a general anesthetic, and recent data suggest that anesthetics can cause neuronal damage when exposure occurs during development. The precise mechanisms are not completely understood. To evaluate the degree of ketamine-induced neuronal toxicity, neural stem cells were isolated from gestational day 16 rat fetuses. On the eighth day in culture, proliferating neural stem cells were exposed for 24 h to ketamine at 1, 10, 100, and 500 μM. To determine the effect of ketamine on differentiated stem cells, separate cultures of neural stem cells were maintained in transition medium (DIV 6) for 1 day and kept in differentiation medium for another 3 days. Differentiated neural cells were exposed for 24 h to 10 μM ketamine. Markers of cellular proliferation and differentiation, mitochondrial health, cell death/damage, and oxidative damage were monitored to determine: (1) the effects of ketamine on neural stem cell proliferation and neural stem cell differentiation; (2) the nature and degree of ketamine-induced toxicity in proliferating neural stem cells and differentiated neural cells; and (3) to provide information regarding receptor expression and possible mechanisms underlying ketamine toxicity. After ketamine exposure at a clinically relevant concentration (10 μM), neural stem cell proliferation was not significantly affected and oxidative DNA damage was not induced. No significant effect on mitochondrial viability (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay) in neural stem cell cultures (growth medium) was observed at ketamine concentrations up to 500 μM. However, quantitative analysis shows that the number of differentiated neurons was substantially reduced in 10 μM ketamine-exposed cultures in differentiation medium, compared with the controls. No significant changes in the number of GFAP-positive astrocytes and O4-positive oligodendrocytes (in differentiation medium) were detected from ketamine-exposed cultures. The discussion focuses on: (1) the doses and time-course over which ketamine is associated with damage of neural cells; (2) how ketamine directs or signals neural stem cells/neural cells to undergo apoptosis or necrosis; (3) how functional neuronal transmitter receptors affect neurotoxicity induced by ketamine; and (4) advantages of using neural stem cell models to study critical issues related to ketamine anesthesia.









Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- l-Ca:
-
Acetyl-l-carnitine
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ELISA:
-
Enzyme-linked immunosorbant assay
- TUNEL:
-
Terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) nick end labeling
- MTT:
-
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
- LDH:
-
Lactate dehydrogenase
References
Deasy C, Babl FE (2010) Intravenous vs intramuscular ketamine for pediatric procedural sedation by emergency medicine specialists: a review. Paediatr Anaesth 20(9):787–796. doi:10.1111/j.1460-9592.2010.03338.x
Muir WW (2010) NMDA receptor antagonists and pain: ketamine. Vet Clin North Am Equine Pract 26(3):565–578. doi:10.1016/j.cveq.2010.07.009
Kohrs R, Durieux ME (1998) Ketamine: teaching an old drug new tricks. Anesth Analg 87(5):1186–1193
Collingridge GL, Kehl SJ, McLennan H (1983) Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 334:33–46
Meldrum B, Garthwaite J (1990) Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 11(9):379–387. doi:10.1016/0165-6147(90)90184-A
D'Souza SW, McConnell SE, Slater P, Barson AJ (1993) Glycine site of the excitatory amino acid N-methyl-D-aspartate receptor in neonatal and adult brain. Arch Dis Child 69(2):212–215
Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ (1996) PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17(3):413–422. doi:10.1016/S0896-6273(00)80174-9
Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA (2001) Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology : Off Publ Am Coll Neuropsychopharmacol 25(4):455–467. doi:10.1016/S0893-133X(01)00243-3
Larsen JO, Gundersen HJ, Nielsen J (1998) Global spatial sampling with isotropic virtual planes: estimators of length density and total length in thick, arbitrarily orientated sections. J Microsc 191(3):238–248
Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ (2006) Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther 316(1):315–324. doi:10.1124/jpet.105.091199
Scallet AC, Schmued LC, Slikker W Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS et al (2004) Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci : Off J Soc Toxicol 81(2):364–370. doi:10.1093/toxsci/kfh224
Slikker W Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC et al (2007) Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci : Off J Soc Toxicol 98(1):145–158. doi:10.1093/toxsci/kfm084
Shi Q, Guo L, Patterson TA, Dial S, Li Q, Sadovova N, Zhang X, Hanig JP et al (2010) Gene expression profiling in the developing rat brain exposed to ketamine. Neuroscience 166(3):852–863. doi:10.1016/j.neuroscience.2010.01.007
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V et al (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283(5398):70–74
Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W Jr et al (2009) Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci : Off J Int Soc Dev Neurosci 27(7):727–731. doi:10.1016/j.ijdevneu.2009.06.010
Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP et al (2009) Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci : Off J Soc Toxicol 108(1):149–158. doi:10.1093/toxsci/kfn270
Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V (2005) Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 135(3):815–827. doi:10.1016/j.neuroscience.2005.03.064
Amy L, Inselman CW, Fang L, and Hansen DK (2014) Handbook of nanotoxicology, nanomedicine and stem cell use in toxicology. Stem cells in toxicity testing in part three: stem cell toxicology. Wiley. doi:10.1002/9781118856017
Wang C, Liu F, Patterson TA, Paule MG, Slikker W Jr (2013) Utilization of neural stem cell-derived models to study anesthesia-related toxicity and preventative approaches. Mol Neurobiol 48(2):302–307. doi:10.1007/s12035-013-8501-z
Wang C, Kaufmann JA, Sanchez-Ross MG, Johnson KM (2000) Mechanisms of N-methyl-D-aspartate-induced apoptosis in phencyclidine-treated cultured forebrain neurons. J Pharmacol Exp Ther 294(1):287–295
Wang C, Fridley J, Johnson KM (2005) The role of NMDA receptor upregulation in phencyclidine-induced cortical apoptosis in organotypic culture. Biochem Pharmacol 69(9):1373–1383. doi:10.1016/j.bcp.2005.02.013
Johnson KM, Phillips M, Wang C, Kevetter GA (1998) Chronic phencyclidine induces behavioral sensitization and apoptotic cell death in the olfactory and piriform cortex. J Neurosci Res 52(6):709–722. doi:10.1002/(SICI)1097-4547(19980615)52:6<709::AID-JNR10>3.0.CO;2-U
Barreto-Chang OL, Dolmetsch RE (2009) Calcium imaging of cortical neurons using Fura-2 AM. J Vis Exp (23). doi:10.3791/1067
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci : Off J Soc Neurosci 23(3):876–882
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA et al (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33(2):220–230. doi:10.1016/j.ntt.2011.01.001
Wang C, Liu F, Patterson TA, Paule MG, Slikker W Jr (2013) Preclinical assessment of ketamine. CNS Neurosci Ther. doi:10.1111/cns.12079
Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W (2005) The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience 132(4):967–977. doi:10.1016/j.neuroscience.2005.01.053
Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG et al (2006) Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci : Off J Soc Toxicol 91(1):192–201. doi:10.1093/toxsci/kfj144
Liu F, Rainosek SW, Sadovova N, Fogle CM, Patterson TA, Hanig JP, Paule MG, Slikker W Jr et al (2014) Protective effect of acetyl-l-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology 42C:49–57. doi:10.1016/j.neuro.2014.03.011
Competing Interests
No external funding and no competing interests declared.
Disclaimer
This document has been reviewed in accordance with United States Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Slikker, W., Liu, F., Rainosek, S.W. et al. Ketamine-Induced Toxicity in Neurons Differentiated from Neural Stem Cells. Mol Neurobiol 52, 959–969 (2015). https://doi.org/10.1007/s12035-015-9248-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9248-5