Abstract
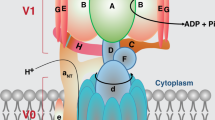
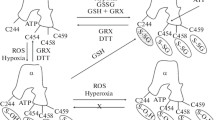
P2C-type ATPases are a subfamily of P-type ATPases comprising Na+/K+-ATPase and H+/K+-ATPase. Na+/K+-ATPase is ubiquitously expressed and has been implicated in several neurological diseases, whereas H+/K+-ATPase is found principally in the colon, stomach, and kidney. Both ATPases have two subunits, α and β, but Na+/K+-ATPase also has a regulatory subunit called FXYD, which has an important role in cancer. The most important functions of these ATPases are homeostasis, potassium regulation, and maintaining a gradient in different cell types, like epithelial cells. Na+/K+-ATPase has become a center of attention ever since it was proposed that it might play a crucial role in neurological disorders such as bipolar disorder, mania, depression, familial hemiplegic migraine, rapid-onset dystonia parkinsonism, chronic stress, epileptogenesis, and Alzheimer’s disease. On the other hand, it has been reported that lithium could have a neuroprotective effect against ouabain, which is the best known Na+/K+-ATPase inhibitor, but and high concentrations of lithium could affect negatively H+/K+-ATPase activity, that has a key role in regulating acidosis and potassium deficiencies. Finally, potassium homeostasis regulation is composed of two main mechanisms, extrarenal and renal. Extrarenal mechanism controls plasma levels, shifting potassium from the extracellular to the intracellular, whereas renal mechanism concerns with body balance and is influenced by potassium intake and its urinary excretion. In this article, we discuss the functions, isoforms, and localization of P2C-type ATPases, describe some of their modulators, and discuss their implications in some diseases.





Similar content being viewed by others
References
Köksoy AA (2002) Na, K-ATPase: a review. J ANKARA Med Sch 24:73–82
Sweadner KJ, Donnet C (2001) Structural similarities of Na, K-ATPase and SERCA, the Ca(2+)-ATPase of the sarcoplasmic reticulum. Biochem J 356:685–704
Abe K, Tani K, Friedrich T, Fujiyoshi Y (2012) Cryo-EM structure of gastric H+, K+-ATPase with a single occupied cation-binding site. Proc Natl Acad Sci U S A 109:18401–18406. doi:10.1073/pnas.1212294109
Nyblom M, Poulsen H, Gourdon P et al (2013) Crystal structure of Na+, K(+)-ATPase in the Na(+)-bound state. Science 342:123–127. doi:10.1126/science.1243352
Li J, Codina J, Petroske E et al (2004) The carboxy terminus of the colonic H(+), K(+)-ATPase alpha-subunit is required for stable beta subunit assembly and function. Kidney Int 65:1301–1310. doi:10.1111/j.1523-1755.2004.00507.x
Toustrup-Jensen MS, Holm R, Einholm AP et al (2009) The C terminus of Na+, K+-ATPase controls Na+ affinity on both sides of the membrane through Arg935. J Biol Chem 284:18715–18725. doi:10.1074/jbc.M109.015099
Mishra NK, Peleg Y, Cirri E et al (2011) FXYD proteins stabilize Na, K-ATPase: amplification of specific phosphatidylserine-protein interactions. J Biol Chem 286:9699–9712. doi:10.1074/jbc.M110.184234
Hall K, Perez G, Sachs G, Rabon E (1991) Identification of H+/K(+)-ATPase alpha, beta-heterodimers. Biochim Biophys Acta 1077:173–179
Chiampanichayakul S, Szekeres A, Khunkaewla P et al (2002) Engagement of Na, K-ATPase beta3 subunit by a specific mAb suppresses T and B lymphocyte activation. Int Immunol 14:1407–1414
Ray T (2013) The parietal cell gastric H, K-ATPase also functions as the Na, K-ATPase and Ca-ATPase in altered states. F1000 Res 2:165–175. doi:10.12688/f1000research.2-165.v2
Shin JM, Munson K, Sachs G (2011) Gastric H+, K+-ATPase. Compr Physiol 1:2141–2153. doi:10.1002/cphy.c110010
Shin JM, Munson K, Vagin O, Sachs G (2009) The gastric HK-ATPase: structure, function, and inhibition. Pflugers Arch 457:609–622. doi:10.1007/s00424-008-0495-4
Al-Khalili L, Kotova O, Tsuchida H et al (2004) ERK1/2 mediates insulin stimulation of Na(+), K(+)-ATPase by phosphorylation of the alpha-subunit in human skeletal muscle cells. J Biol Chem 279:25211–25218. doi:10.1074/jbc.M402152200
Cornelius F, Mahmmoud YA (2003) Direct activation of gastric H, K-ATPase by N-terminal protein kinase C phosphorylation. Comparison of the acute regulation mechanisms of H, K-ATPase and Na, K-ATPase. Biophys J 84:1690–1700. doi:10.1016/S0006-3495(03)74977-7
Poulsen H, Morth P, Egebjerg J, Nissen P (2010) Phosphorylation of the Na+, K+−ATPase and the H+, K+−ATPase. FEBS Lett 584:2589–2595. doi:10.1016/j.febslet.2010.04.035
Mahmmoud YA, Cornelius F (2003) PKA and PKC phosphorylation of gastric H, K-ATPase. Ann N Y Acad Sci 986:548–549
Frindt G, Palmer LG (2012) Effects of insulin on Na and K transporters in the rat CCD. Am J Physiol Renal Physiol 302:F1227–F1233. doi:10.1152/ajprenal.00675.2011
Aperia A (2007) New roles for an old enzyme: Na, K-ATPase emerges as an interesting drug target. J Intern Med 261:44–52. doi:10.1111/j.1365-2796.2006.01745.x
Xie JX, Li X, Xie Z (2013) Regulation of renal function and structure by the signaling Na/K-ATPase. IUBMB Life 65:991–998. doi:10.1002/iub.1229
Lingrel JB, Orlowski J, Shull MM, Price EM (1990) Molecular genetics of Na, K-ATPase. Prog Nucleic Acid Res Mol Biol 38:37–89
Wang H-YL, O’Doherty GA (2012) Modulators of Na/K-ATPase: a patent review. Expert Opin Ther Pat 22:587–605. doi:10.1517/13543776.2012.690033
Li H, Meng L, Liu F et al (2013) H+/K+−ATPase inhibitors: a patent review. Expert Opin Ther Pat 23:99–111. doi:10.1517/13543776.2013.741121
Beaugé L (1978) Activation by lithium ions of the inside sodium sites in Na, K-ATPase. Niochimica Biophys Acta 527:472–484
Dafnis E, Kurtzman NA, Sabatini S (1993) H-K-ATPase in distal renal tubular acidosis: urinary tract obstruction, lithium, and amiloride. Am J Physiol 265:F875–F880
Crowson MS, Shull GE (1992) Isolation and characterization of a cDNA encoding the putative distal colon H+, K(+)-ATPase. Similarity of deduced amino acid sequence to gastric H+, K(+)-ATPase and Na+, K(+)-ATPase and mRNA expression in distal colon, kidney, and uterus. J Biol Chem 267:13740–13748
Horisberger JD, Lemas V, Kraehenbühl JP, Rossier BC (1991) Structure-function relationship of Na, K-ATPase. Annu Rev Physiol 53:565–584. doi:10.1146/annurev.ph.53.030191.003025
Kaplan JH (2002) Biochemistry of Na, K-ATPase. Annu Rev Biochem 71:511–535. doi:10.1146/annurev.biochem.71.102201.141218
Apell H-J (2004) How do P-type ATPases transport ions? Bioelectrochemistry 63:149–156. doi:10.1016/j.bioelechem.2003.09.021
Herrera VL, Emanuel JR, Ruiz-Opazo N et al (1987) Three differentially expressed Na, K-ATPase alpha subunit isoforms: structural and functional implications. J Cell Biol 105:1855–1865
Woo AL, James PF, Lingrel JB (1999) Characterization of the Fourth alpha Isoform of the Na, K-ATPase. J Membr Biol 169:39–44
McDermott JP, Sánchez G, Chennathukuzhi V, Blanco G (2012) Green fluorescence protein driven by the Na, K-ATPase α4 isoform promoter is expressed only in male germ cells of mouse testis. J Assist Reprod Genet 29:1313–1325. doi:10.1007/s10815-012-9876-x
Jimenez T, Sánchez G, Wertheimer E, Blanco G (2010) Activity of the Na, K-ATPase alpha4 isoform is important for membrane potential, intracellular Ca2+, and pH to maintain motility in rat spermatozoa. Reproduction 139:835–845. doi:10.1530/REP-09-0495
Jimenez T, Mcdermott JP, Sánchez G, Blanco G (2010) Na, K-ATPase α4 isoform is essential for sperm fertility. Proc Natl Acad Sci U S A 108:644–649. doi:10.1073/pnas.1016902108/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1016902108
Lingrel JB, Williams MT, Vorhees CV, Moseley AE (2007) Na, K-ATPase and the role of alpha isoforms in behavior. J Bioenerg Biomembr 39:385–389. doi:10.1007/s10863-007-9107-9
Kirshenbaum GS, Clapcote SJ, Duffy S et al (2011) Mania-like behavior induced by genetic dysfunction of the neuron-specific Na, K-ATPase α3 sodium pump. Proc Natl Acad Sci U S A 108:18144–188149. doi:10.1073/pnas.1108416108/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1108416108
Edwards IJ, Bruce G, Lawrenson C et al (2013) Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α- and γ-motoneurons. J Neurosci 33:9913–9919. doi:10.1523/JNEUROSCI. 5584-12.2013
Despa S, Lingrel JB, Bers DM (2012) Na(+)/K)+)-ATPase α2-isoform preferentially modulates Ca2(+) transients and sarcoplasmic reticulum Ca2(+) release in cardiac myocytes. Cardiovasc Res 95:480–486. doi:10.1093/cvr/cvs213
Clifford RJ, Kaplan JH (2008) B-Subunit overexpression alters the stoicheometry of assembled Na-K-ATPase subunits in MDCK cells. Am J Physiol Ren Physiol 295:1314–1323. doi:10.1152/ajprenal.90406.2008
Chow DARC, Forte JG (1995) Functional significance of the beta subunit for heterodimeric P-type ATPases. J Exp Biol 198:1–17
McDonough AA, Geering K, Farley RA (1990) The sodium pump needs its beta subunit. FASEB J 4:1598–1605
Vagin O, Sachs G, Tokhtaeva E (2007) The roles of the Na, K-ATPase beta 1 subunit in pump sorting and epithelial integrity. J Bioenerg Biomembr 39:367–372. doi:10.1007/s10863-007-9103-0
Sun MZ, Kim JM, Oh MC et al (2013) Na+/K+-ATPase β2-subunit (AMOG) expression abrogates invasion of glioblastoma-derived brain tumor-initiating cells. Neuro Oncol 15:1518–1531. doi:10.1093/neuonc/not099
Peng L, Martin-Vasallo P, Sweadner KJ (1997) Isoforms of Na, K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures. J Neurosci 17:3488–3502
Azarias G, Kruusmägi M, Connor S et al (2013) A specific and essential role for Na, K-ATPase α3 in neurons co-expressing α1 and α3. J Biol Chem 288:2734–2743. doi:10.1074/jbc.M112.425785
Moseley AE, Williams MT, Schaefer TL et al (2007) Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci 27:616–626. doi:10.1523/JNEUROSCI. 4464-06.2007
Geering K (2005) Function of FXYD proteins, regulators of Na, K-ATPase. J Bioenerg Biomembr 37:387–392. doi:10.1007/s10863-005-9476-x
Mijatovic T, Ingrassia L, Facchini V, Kiss R (2008) Na+/K+−ATPase alpha subunits as new targets in anticancer therapy. Expert Opin Ther Targets 12:1403–1417. doi:10.1517/14728222.12.11.1403
Salhi A, Lamouroux C, Pestov NB et al (2013) A link between fertility and K+ homeostasis: role of the renal H, K-ATPase type 2. Pflugers Arch 465:1149–1158. doi:10.1007/s00424-013-1252-x
Streif D, Iglseder E, Hauser-kronberger C et al (2011) Expression of the non-gastric H+/K+−ATPase ATP12A in normal and pathological human prostate tissue. Cell Physiol Biochem 28:1287–1294
Cougnon M, Bouyer P, Planelles G, Jaisser F (1998) Does the colonic H, K-ATPase also act as an Na, K-ATPase? Proc Natl Acad Sci U S A 95:6516–6520
Codina J (1999) The colonic H+, K+-ATPase functions as a Na+-dependent K+(NH4+)-ATPase in apical membranes from rat distal colon. J Biol Chem 274:19693–19698. doi:10.1074/jbc.274.28.19693
Gumz ML, Lynch IJ, Greenlee MM et al (2010) The renal H+-K+-ATPases: physiology, regulation, and structure. Am J Physiol Ren Physiol 298:12–21. doi:10.1152/ajprenal.90723.2008
Zies DL, Gumz ML, Wingo CS, Cain BD (2007) The renal H+, K+-ATPases as therapeutic targets. Expert Opin Ther Targets 11:881–890. doi:10.1517/14728222.11.7.881
Fryklund J, Wallmark B, Larsson H, Helander HF (1984) Effect of omeprazole on gastric secretion in H+, K+-ATPase and in pepsinogen-rich cell fractions from rabbit gastric mucosa. Biochem Pharmacol 33:273–280
Belisario DC, Rocafull MA, del Castillo JR (2010) Purification and characterization of the ouabain-sensitive H+/K+-ATPase from guinea-pig distal colon. Arch Biochem Biophys 496:21–32. doi:10.1016/j.abb.2010.01.014
Codina J (1998) The alpha subunit of the colonic H+, K+-ATPase assembles with beta 1-Na+, K+-ATPase in kidney and distal colon. J Biol Chem 273:7894–7899. doi:10.1074/jbc.273.14.7894
Bamford M (2009) 3H+/K+ ATPase inhibitors in the treatment of acid-related disorders. Prog Med Chem 47:75–162. doi:10.1016/S0079-6468(08)00203-8
Yatime L, Buch-Pedersen MJ, Musgaard M et al (2009) P-type ATPases as drug targets: tools for medicine and science. Biochim Biophys Acta Bioenerg 1787:207–220. doi:10.1016/j.bbabio.2008.12.019
Rajendran VM (2000) Ouabain-sensitive H, K-ATPase functions as Na, K-ATPase in apical membranes of rat distal colon. J Biol Chem 275:13035–13040. doi:10.1074/jbc.275.17.13035
Sangan P, Thevananther S, Sangan S et al (2000) Colonic H-K-ATPase alpha- and beta-subunits express ouabain-insensitive H-K-ATPase. Am J Physiol Cell Physiol 278:C182–C189
Bhattacharyya D, Sen PC (1999) The effect of binding of chlorpromazine and chloroquine to ion transporting ATPases. Mol Cell Biochem 198:179–185
Gupta SP (2012) Quantitative structure-activity relationship studies on Na+, K(+)-ATPase inhibitors. Chem Rev 112:3171–3192. doi:10.1021/cr200097p
Sandtner W, Egwolf B, Khalili-Araghi F et al (2011) Ouabain binding site in a functioning Na+/K+ ATPase. J Biol Chem 286:38177–38183. doi:10.1074/jbc.M111.267682
Yu SP (2003) Na+, K+-ATPase: the new face of an old player in pathogenesis and apoptotic/hybrid cell death. Biochem Pharmacol 66:1601–1609. doi:10.1016/S0006-2952(03)00531-8
Goldstein I, Levy T, Galili D et al (2006) Involvement of Na(+), K(+)-ATPase and endogenous digitalis-like compounds in depressive disorders. Biol Psychiatr 60:491–499. doi:10.1016/j.biopsych.2005.12.021
Herman L, Hougland T, El-Mallakh RS (2007) Mimicking human bipolar ion dysregulation models mania in rats. Neurosci Biobehav Rev 31:874–881. doi:10.1016/j.neubiorev.2007.04.001
Rosta K, Tulassay E, Enzsoly A et al (2009) Insulin induced translocation of Na+/K+-ATPase is decreased in the heart of streptozotocin diabetic rats. Acta Pharmacol Sin 30:1616–1624. doi:10.1038/aps.2009.162
Oubaassine R, Weckering M, Kessler L et al (2012) Insulin interacts directly with Na+/K+ATPase and protects from digoxin toxicity. Toxicology 299:1–9. doi:10.1016/j.tox.2012.04.013
Haritha C, Reddy AG, Reddy YR et al (2013) Evaluation of protective action of fenugreek, insulin and glimepiride and their combination in diabetic Sprague Dawley rats. J Nat Sci Biol Med 4:207–212. doi:10.4103/0976-9668.107292
Liu J, Feng L, Zhang M et al (2013) Neuroprotective effect of Liuwei Dihuang decoction on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. J Ethnopharmacol 150:371–381. doi:10.1016/j.jep.2013.09.003
Hennion JP, el-Masri MA, Huff MO, el-Mailakh RS (2002) Evaluation of neuroprotection by lithium and valproic acid against ouabain-induced cell damage. Bipolar Disord 4:201–206
El-Mallakh RS, Schurr A, Payne RS, Li R (2000) Ouabain induction of cycling of multiple spike responses in hippocampal slices is delayed by lithium. J Psychiatr Res 34:115–120
Banerjee U, Dasgupta A, Rout JK, Singh OP (2012) Effects of lithium therapy on Na+−K+-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatr 37:56–61. doi:10.1016/j.pnpbp.2011.12.006
De Vasconcellos APS, Zugno AI, Dos Santos AHDP et al (2005) Na+, K(+)-ATPase activity is reduced in hippocampus of rats submitted to an experimental model of depression: effect of chronic lithium treatment and possible involvement in learning deficits. Neurobiol Learn Mem 84:102–110. doi:10.1016/j.nlm.2005.05.002
McKnight RF, Adida M, Budge K et al (2012) Lithium toxicity profile: a systematic review and meta-analysis. Lancet 379:721–728. doi:10.1016/S0140-6736(11)61516-X
Wu JY, Wadhwa N (2013) Case 192: lithium-induced nephropathy. Radiology 267:308–312. doi:10.1148/radiol.13111801
Rej S, Looper K, Segal M (2013) The effect of serum lithium levels on renal function in geriatric outpatients: a retrospective longitudinal study. Drugs Aging 30:409–415. doi:10.1007/s40266-013-0068-x
Andrade Nunes M, Araujo Viel T, Sousa Buck H (2013) Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res 10:104–107. doi:10.2174/156720513804871354
Nunes PV, Forlenza OV, Gattaz WF (2007) Lithium and risk for Alzheimer’s disease in elderly patients with bipolar disorder. Br J Psychiatry 190:359–360. doi:10.1192/bjp.bp.106.029868
Goldstein I, Lerer E, Laiba E et al (2009) Association between sodium- and potassium-activated adenosine triphosphatase alpha isoforms and bipolar disorders. Biol Psychiatry 65:985–991. doi:10.1016/j.biopsych.2008.10.033
Bøttger P, Doğanlı C, Lykke-Hartmann K (2012) Migraine- and dystonia-related disease-mutations of Na+/K+-ATPases: relevance of behavioral studies in mice to disease symptoms and neurological manifestations in humans. Neurosci Biobehav Rev 36:855–871. doi:10.1016/j.neubiorev.2011.10.005
Gritz SM, Radcliffe RA (2013) Genetic effects of ATP1A2 in familial hemiplegic migraine type II and animal models. Hum Genom 7:8. doi:10.1186/1479-7364-7-8
Heinzen EL, Arzimanoglou A, Brashear A et al (2014) Distinct neurological disorders with ATP1A3 mutations. Lancet Neurol 13:503–514. doi:10.1016/S1474-4422(14)70011-0
De Carvalho AP, Sweadner KJ, Penniston JT et al (2004) Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43:169–175. doi:10.1016/j.neuron.2004.06.028
Brashear A, Mink JW, Hill DF et al (2012) ATP1A3 mutations in infants: a new rapid-onset dystonia-Parkinsonism phenotype characterized by motor delay and ataxia. Dev Med Child Neurol 54:1065–1067. doi:10.1111/j.1469-8749.2012.04421.x
Einholm AP, Toustrup-Jensen MS, Holm R et al (2010) The rapid-onset dystonia parkinsonism mutation D923N of the Na+, K+-ATPase alpha3 isoform disrupts Na+ interaction at the third Na+ site. J Biol Chem 285:26245–26254. doi:10.1074/jbc.M110.123976
Heinzen EL, Swoboda KJ, Hitomi Y et al (2012) De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet 44:1030–1034. doi:10.1038/ng.2358
Hattori N, Kitagawa K, Higashida T et al (1998) Cl-ATPase and Na, K-ATPase activities in Alzheimer’s disease brains. Neurosci Lett 254:141–144
Toustrup-Jensen MS, Einholm AP, Schack VR et al (2013) Relationship between intracellular Na+ concentration and reduced Na+ affinity in Na+, K+-ATPase mutants causing neurological disease. J Biol Chem. doi:10.1074/jbc.M113.543272
Silveira PP, Portella AK, Benetti CDS et al (2011) Association between Na+, K+-ATPase activity and the vulnerability/resilience to mood disorders induced by early life experience. Neurochem Res 36:2075–2082. doi:10.1007/s11064-011-0531-1
Kirshenbaum GS, Clapcote SJ, Petersen J et al (2012) Genetic suppression of agrin reduces mania-like behavior in Na+, K+−ATPase α3 mutant mice. Genes Brain Behav 11:436–443. doi:10.1111/j.1601-183X.2012.00800.x
Harik SI, Mitchell MJ, Kalaria RN (1989) Ouabain binding in the human brain. Effects of Alzheimer’s disease and aging. Arch Neurol 46:951–954
Mark J, Allan D, Mattson P (1995) Amyloid P-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci 15:6239–6249
Kairane C, Mahlapuu R, Ehrlich K et al (2014) The effects of different antioxidants on the activity of cerebrocortical MnSOD and Na, K-ATPase from post mortem Alzheimer’s disease and age-matched normal brains. Curr Alzheimer Res 11:79–85. doi:10.2174/15672050113106660179
Wilson M-MG, Morley JE (2003) Invited review: aging and energy balance. J Appl Physiol 95:1728–1736. doi:10.1152/japplphysiol.00313.2003
Gu QB, Zhao JX, Fei J, Schwarz W (2004) Modulation of Na(+), K(+) pumping and neurotransmitter uptake by beta-amyloid. Neuroscience 126:61–67. doi:10.1016/j.neuroscience.2004.03.022
Dickey CA, Gordon MN, Wilcock DM et al (2005) Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice. BMC Neurosci 6:7. doi:10.1186/1471-2202-6-7
Zhang L-N, Sun Y-J, Pan S et al (2013) Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol 27:96–103. doi:10.1111/fcp.12000
Kreutz F, Scherer EB, Ferreira AGK et al (2013) Alterations on Na+, K+-ATPase and acetylcholinesterase activities induced by amyloid-β peptide in rat brain and GM1 ganglioside neuroprotective action. Neurochem Res 38:2342–2350. doi:10.1007/s11064-013-1145-6
Eiam-ong S, Jerawatana R, Kittikowit W, Mannontarat R (2009) Effects of vanadate and potassium depletion on renal H. K-ATPase Protein Expr 3:517–523
Codina J, Delmas-Mata JT, DuBose TD (1998) Expression of HKalpha2 protein is increased selectively in renal medulla by chronic hypokalemia. Am J Physiol 275:F433–F440
Izumi Y, Hori K, Nakayama Y et al (2011) Aldosterone requires vasopressin V1a receptors on intercalated cells to mediate acid–base homeostasis. J Am Soc Nephrol 22:673–680. doi:10.1681/ASN.2010050468
Salhi A, Centeno G, Firsov D, Crambert G (2012) Circadian expression of H, K-ATPase type 2 contributes to the stability of plasma K+ levels. FASEB J 26:2859–2867. doi:10.1096/fj.11-199711
Maeda Y, Kojima N, Araki Y et al (2011) Does a proton pump inhibitor cause hypokalemia? Intern Med 50:1045–1050. doi:10.2169/internalmedicine.50.4877
Vío CP, Figueroa CD (1987) Evidence for a stimulatory effect of high potassium diet on renal kallikrein. Kidney Int 31:1327–1334
Vío CP, Figueroa CD (1985) Subcellular localization of renal kallikrein by ultrastructural immunocytochemistry. Kidney Int 28:36–42
Valdés G, Vio CP, Montero J, Avendaño R (1991) Potassium supplementation lowers blood pressure and increases urinary kallikrein in essential hypertensives. J Hum Hypertens 5:91–96
Suga SI, Phillips MI, Ray PE et al (2001) Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol 281:F620–F629
He FJ, MacGregor GA (2001) Fortnightly review: beneficial effects of potassium. BMJ 323:497–501
Aburto NJ, Hanson S, Gutierrez H et al (2013) Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 346:f1378. doi:10.1136/bmj.f1378
McDonough AA, Nguyen MTX (2012) How does potassium supplementation lower blood pressure? Am J Physiol Renal Physiol 302:F1224–F1225. doi:10.1152/ajprenal.00429.2011
Ogiyama Y, Miura T, Watanabe S, et al (2013) Circadian rhythm of urinary potassium excretion during treatment with an angiotensin receptor blocker. J Renin Angiotensin Aldosterone Syst:1–6. doi: 10.1177/1470320313475909
Acknowledgments
This work was supported by grants from the Basal Center of Excellence in Aging and Regeneration (CONICYT-PFB 12/2007) to NCI and CV, FONDECYT No. 1120156 to NCI and FONDECYT No. 1130741. Rocío Retamales-Ortega was a SQM associate researcher.
We thank the Sociedad Química y Minera de Chile (SQM) for grants on the “Role of Potassium in Hypertension and Cognition” and on “The Effect of Lithium on Human Health” to the CARE Biomedical Center.
Graphic work was carried out using Graphique-Science (http://graphiquescience.blogspot.com). We thank Felipe Serrano for his help with the drawing of our figures.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Retamales-Ortega, R., Vio, C.P. & Inestrosa, N.C. P2C-Type ATPases and Their Regulation. Mol Neurobiol 53, 1343–1354 (2016). https://doi.org/10.1007/s12035-014-9076-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9076-z