Abstract
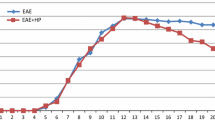
The protective and therapeutic mechanism of bee venom acupuncture (BVA) in neurodegenerative disorders is not clear. We investigated whether treatment with BVA (0.25 and 0.8 mg/kg) at the Zusanli (ST36) acupoints, located lateral from the anterior border of the tibia, has a beneficial effect in a myelin basic protein (MBP)68–82-induced acute experimental autoimmune encephalomyelitis (EAE) rat model. Pretreatment (every 3 days from 1 h before immunization) with BVA was more effective than posttreatment (daily after immunization) with BVA with respect to clinical signs (neurological impairment and loss of body weight) of acute EAE rats. Treatment with BVA at the ST36 acupoint in normal rats did not induce the clinical signs. Pretreatment with BVA suppressed demyelination, glial activation, expression of cytokines [interferon (IFN)-γ, IL-17, IL-17A, tumor necrosis factor-alpha (TNF-α), and IL-1β], chemokines [RANTES, monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory protein (MIP)-1α], and inducible nitric oxide synthase (iNOS), and activation of p38 mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB (p65 and phospho-IκBα) signaling pathways in the spinal cord of acute EAE rats. Pretreatment with BVA decreased the number of CD4+, CD4+/IFN-γ+, and CD4+/IL-17+ T cells, but increased the number of CD4+/Foxp3+ T cells in the spinal cord and lymph nodes of acute EAE rats. Treatment with BVA at six placebo acupoints (SP9, GB39, and four non-acupoints) did not have a positive effect in acute EAE rats. Interestingly, onset and posttreatment with BVA at the ST36 acupoint markedly attenuated neurological impairment in myelin oligodendrocyte glycoprotein (MOG)35–55-induced chronic EAE mice compared to treatment with BVA at six placebo acupoints. Our findings strongly suggest that treatment with BVA with ST36 acupoint could delay or attenuate the development and progression of EAE by upregulating regulatory T cells and suppressing T-helper (Th) 17 and Th1 responses. These results warrant further investigation of BVA as a treatment for autoimmune disorders of the central nervous system.













Similar content being viewed by others
References
Lassmann H, van Horssen J (2011) The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 585:3715–3723
McFarland HF, Martin R (2007) Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8:913–919
Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P, Andreoni L, Trivedi P, Salvetti M, Faggioni A, Aloisi F (2007) Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med 204:2899–2912
Ragheb S, Lisak R (1993) Multiple sclerosis: genetic background versus environment. Ann Neurol 34:509–510
Madsen LS, Andersson EC, Jansson L, Krogsgaard M, Andersen CB, Engberg J, Strominger JL, Svejgaard A, Hjorth JP, Holmdahl R, Wucherpfennig KW, Fugger L (1999) A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet 23:343–347
Benveniste EN (1997) Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med (Berl) 75:165–173
Engelhardt B (2006) Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm 113:477–485
Merrill JE, Benveniste EN (1996) Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 19:331–338
Murphy, R.P., Murphy, K.J., Pickering, M., 2012. The development of myelin repair agents for treatment of multiple sclerosis: progress and challenges. Bioengineered 4
Mahdavian S, Dike U, Bryant A, Davison C, Ghazvini P, Hill A (2010) Multiple sclerosis: a supplement on the disease state, current therapies, and investigational treatments. J Pharm Pract 23:91–100
Chen J, Lariviere WR (2010) The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword. Prog Neurobiol 92:151–183
Bilò MB, Antonicelli L, Bonifazi F. (2012) Honeybee venom immunotherapy: certainties and pitfalls. Immunotherapy 4(11):1153–1166. doi:10.2217/imt.12.113
Kavoussi B, Ross BE (2007) The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther 6:251–257
Kang SY, Kim CY, Roh DH, Yoon SY, Park JH, Lee HJ, Beitz AJ, Lee JH (2011) Chemical stimulation of the ST36 acupoint reduces both formalin-induced nociceptive behaviors and spinal astrocyte activation via spinal alpha-2 adrenoceptors. Brain Res Bull 86:412–421
Yoon SY, Roh DH, Kwon YB, Kim HW, Seo HS, Han HJ, Lee HJ, Beitz AJ, Lee JH (2009) Acupoint stimulation with diluted bee venom (apipuncture) potentiates the analgesic effect of intrathecal clonidine in the rodent formalin test and in a neuropathic pain model. J Pain 10:253–263
Lee JY, Kang SS, Kim JH, Bae CS, Choi SH (2005) Inhibitory effect of whole bee venom in adjuvant-induced arthritis. In Vivo 19:801–805
Kwon YB, Lee HJ, Han HJ, Mar WC, Kang SK, Yoon OB, Beitz AJ, Lee JH (2002) The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci 71:191–204
Shinto, L., Calabrese, C., Morris, C., Sinsheimer, S., Bourdette, D., 2004. Complementary and alternative medicine in multiple sclerosis: survey of licensed naturopaths. J Altern Complement Med (New York, NY) 10, 891–897.
Wesselius T, Heersema DJ, Mostert JP, Heerings M, Admiraal-Behloul F, Talebian A, van Buchem MA, De Keyser J (2005) A randomized crossover study of bee sting therapy for multiple sclerosis. Neurology 65:1764–1768
Stuhlmeier KM (2007) Apis mellifera venom and melittin block neither NF-kappa B-p50-DNA interactions nor the activation of NF-kappa B, instead they activate the transcription of proinflammatory genes and the release of reactive oxygen intermediates. J Immunol 179:655–664
Lee MS, Pittler MH, Shin BC, Kong JC, Ernst E (2008) Bee venom acupuncture for musculoskeletal pain: a review. J Pain 9:289–297
Joos, S., Schott, C., Zou, H., Daniel, V., Martin, E., 2000. Immunomodulatory effects of acupuncture in the treatment of allergic asthma: a randomized controlled study. J Altern Complement Med (New York, NY) 6, 519–525.
Yang EJ, Jiang JH, Lee SM, Yang SC, Hwang HS, Lee MS, Choi SM (2010) Bee venom attenuates neuroinflammatory events and extends survival in amyotrophic lateral sclerosis models. J Neuroinflammation 7:69
Cho SY, Shim SR, Rhee HY, Park HJ, Jung WS, Moon SK, Park JM, Ko CN, Cho KH, Park SU (2012) Effectiveness of acupuncture and bee venom acupuncture in idiopathic Parkinson’s disease. Parkinsonism Relat Disord 18:948–952
Doo AR, Kim SN, Kim ST, Park JY, Chung SH, Choe BY, Chae Y, Lee H, Yin CS, Park HJ (2012) Bee venom protects SH-SY5Y human neuroblastoma cells from 1-methyl-4-phenylpyridinium-induced apoptotic cell death. Brain Res 1429:106–115
Doo AR, Kim ST, Kim SN, Moon W, Yin CS, Chae Y, Park HK, Lee H, Park HJ (2010) Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurol Res 32(Suppl 1):88–91
Kim JI, Yang EJ, Lee MS, Kim YS, Huh Y, Cho IH, Kang S, Koh HK (2011) Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. Int J Neurosci 121:209–217
Kim J, Kang DI (2010) A descriptive statistical approach to the Korean pharmacopuncture therapy. J Acupunc Meridian Stud 3:141–149
Nam MH, Yin CS, Soh KS, Choi SH (2011) Adult neurogenesis and acupuncture stimulation at ST36. J Acupunct Meridian Stud 4:153–158
Zhou Y, Jin J (2008) Effect of acupuncture given at the HT 7, ST 36, ST 40 and KI 3 acupoints on various parts of the brains of Alzheimer’s disease patients. Acupunct Electrother Res 33:9–17
Lassmann H, Bruck W, Lucchinetti C (2001) Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med 7:115–121
Almolda B, Costa M, Montoya M, Gonzalez B, Castellano B (2009) CD4 microglial expression correlates with spontaneous clinical improvement in the acute Lewis rat EAE model. J Neuroimmunol 209:65–80
Mohamed A, Tarhuni H, Dufan T, Benghuzzi H, Tucci M (2004) The use of digital technology to asses the severity of the experimental allergic encephalomyelitis (EAE) spinal cord lesion. Biomed Sci Instrum 40:419–423
Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD (2012) A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490:187–191
Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R (2007) Inflammation in Parkinson’s diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des 13:1925–1928
Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG (2008) A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci 84:159–165
Chang KH, Bai SJ, Lee H, BH L (2014) Effects of acupuncture stimulation at different acupoints on formalin-induced pain in rats. Korean J Physiol Pharmacol 18:121–127
Napadow V, Lee J, Kim J, Cina S, Maeda Y, Barbieri R, Harris RE, Kettner N, Park K (2013) Brain correlates of phasic autonomic response to acupuncture stimulation: an event-related fMRI study. Hum Brain Mapp 34:2592–2606
Watanabe M, Takayama S, Hirano A, Seki T, Yaegashi N (2012) Hemodynamic changes in the brachial artery induced by acupuncture stimulation on the lower limbs: a single-blind randomized controlled trial. Evid Based Complement Alternat Med 2012:958145
Tanahashi N, Shikami J, Yoneda M, Ishida T (2011) Effects of manual acupuncture at GB34 on carbon tetrachloride-induced acute liver injury in rats. J Acupunct Meridian Stud 4:214–219
Hwang HS, Kim YS, Ryu YH, Lee JE, Lee YS, Yang EJ, Choi SM, Lee MS (2011) Electroacupuncture delays hypertension development through enhancing NO/NOS activity in spontaneously hypertensive rats. Evid Based Complement Alternat Med 2011:130529
Yang CH, Yoon SS, Hansen DM, Wilcox JD, Blumell BR, Park JJ, Steffensen SC (2010) Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcohol Clin Exp Res 34:2137–2146
Zhao Z, Jin X, Wu Y, Yang X, Xu Y, Jiang JZ, Kim SC, Lee BH, Yang CH, Zhao R (2013) Amygdaloid corticotropin-releasing factor is involved in the anxiolytic effect of acupuncture during ethanol withdrawal in rats. J Acupunct Meridian Stud 6:234–240
Fissolo N, Costa C, Nurtdinov RN, Bustamante MF, Llombart V, Mansilla MJ, Espejo C, Montalban X, Comabella M (2012) Treatment with MOG-DNA vaccines induces CD4+CD25+FoxP3+ regulatory T cells and up-regulates genes with neuroprotective functions in experimental autoimmune encephalomyelitis. J Neuroinflammation 9:139. doi:10.1186/1742-2094-9-139
Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ (2008) Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain 131:3019–3033
VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM (2011) Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. J Neuroinflammation 8:138
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C (T)) method. Methods 25:402–408
Hong J, Cho IH, Kwak KI, Suh EC, Seo J, Min HJ, Choi SY, Kim CH, Park SH, Jo EK, Lee S, Lee KE, Lee SJ (2011) Microglial Toll-like receptor 2 contributes to kainic acid-induced glial activation and hippocampal neuronal cell death. J Biol Chem 285:39447–39457
Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A 88:7438–7442
Lee HS, Schlereth S, Khandelwal P, Saban DR (2013) Ocular allergy modulation to hi-dose antigen sensitization is a Treg-dependent process. PLoS One 8:e75769
Block ML, Hong JS (2007) Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans 35:1127–1132
Lobsiger CS, Cleveland DW (2007) Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 10:1355–1360
Babcock AA, Toft-Hansen H, Owens T (2008) Signaling through MyD88 regulates leukocyte recruitment after brain injury. J Immunol 181:6481–6490
El-behi M, Rostami A, Ciric B (2010) Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol 5:189–197
Putheti P, Soderstrom M, Link H, Huang YM (2003) Effect of glatiramer acetate (Copaxone) on CD4+CD25high T regulatory cells and their IL-10 production in multiple sclerosis. J Neuroimmunol 144:125–131
Kennedy KJ, Strieter RM, Kunkel SL, Lukacs NW, Karpus WJ (1998) Acute and relapsing experimental autoimmune encephalomyelitis are regulated by differential expression of the CC chemokines macrophage inflammatory protein-1alpha and monocyte chemotactic protein-1. J Neuroimmunol 92:98–108
Hilliard B, Samoilova EB, Liu TS, Rostami A, Chen Y (1999) Experimental autoimmune encephalomyelitis in NF-kappa B-deficient mice: roles of NF-kappa B in the activation and differentiation of autoreactive T cells. J Immunol 163:2937–2943
Noubade R, Krementsov DN, Del Rio R, Thornton T, Nagaleekar V, Saligrama N, Spitzack A, Spach K, Sabio G, Davis RJ, Rincon M, Teuscher C (2011) Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood 118:3290–3300
Mirshafiey A (2007) Venom therapy in multiple sclerosis. Neuropharmacology 53:353–361
Correa JO, Aarestrup BJ, Aarestrup FM (2010) Effect of thalidomide and pentoxifylline on experimental autoimmune encephalomyelitis (EAE). Exp Neurol 226:15–23
Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT (2007) Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther 115:246–270
Liu CZ, Xie JP, Wang LP, Zheng YY, Ma ZB, Yang H, Chen X, Shi GX, Li SL, Zhao JP, Han JX, Li JD, Wang YX, Tang L, Xue XO, Li M, Wang Y, Sun AP, Xing JM, Cao HJ, Zhu J, Liu JP (2011) Immediate analgesia effect of single point acupuncture in primary dysmenorrhea: a randomized controlled trial. Pain Medicine (Malden, Mass) 12:300–307
Yu, Y.P., Ma, L.X., Ma, Y.X., Ma, Y.X., Liu, Y.Q., Liu, C.Z., Xie, J.P., Gao, S.Z., Zhu, J., 2010. Immediate effect of acupuncture at Sanyinjiao (SP6) and Xuanzhong (GB39) on uterine arterial blood flow in primary dysmenorrhea. J Altern Complement Med (New York, NY) 16, 1073–1078.
Kang KA, Shin ES, Hur J, Hasan MR, Lee H, Park HJ, Park HK, Kim YJ (2010) Acupuncture attenuates neuronal cell death in middle cerebral artery occlusion model of focal ischemia. Neurol Res 32(Suppl 1):84–87
Kim ST, Moon W, Chae Y, Kim YJ, Lee H, Park HJ (2010) The effect of electroaucpuncture for 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced proteomic changes in the mouse striatum. J Physiol Sci 60:27–34
Lee GJ, Yin CS, Choi SK, Choi S, Yang JS, Lee H, Park HK (2010) Acupuncture attenuates extracellular glutamate level in global ischemia model of rat. Neurol Res 32(Suppl 1):79–83
Cha MH, Bai SJ, Lee KH, Cho ZH, Kim YB, Lee HJ, Lee BH (2010) Acute electroacupuncture inhibits nitric oxide synthase expression in the spinal cord of neuropathic rats. Neurol Res 32(Suppl 1):96–100
Kim KW, Kim HW, Li J, Kwon YB (2011) Effect of bee venom acupuncture on methamphetamine-induced hyperactivity, hyperthermia and Fos expression in mice. Brain Res Bull 84:61–68
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801
Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM (2003) Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. Proc Natl Acad Sci U S A 100:10483–10487
Ryu JK, Kim SU, McLarnon JG (2004) Blockade of quinolinic acid-induced neurotoxicity by pyruvate is associated with inhibition of glial activation in a model of Huntington’s disease. Exp Neurol 187:150–159
Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC (2007) Loss of blood-brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models. Proc Natl Acad Sci U S A 104:5656–5661
Zeng Y, Gu B, Ji X, Ding X, Song C, Wu F (2007) Sinomenine, an antirheumatic alkaloid, ameliorates clinical signs of disease in the Lewis rat model of acute experimental autoimmune encephalolmyelitis. Biol Pharm Bull 30:1438–1444
Chen SJ, Wang YL, Fan HC, Lo WT, Wang CC, Sytwu HK (2012) Current status of the immunomodulation and immunomediated therapeutic strategies for multiple sclerosis. Clin Dev Immunol 2012:970789
Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK (2004) Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 101:15434–15439
Wraith DC, Nicolson KS, Whitley NT (2004) Regulatory CD4+ T cells and the control of autoimmune disease. Curr Opin Immunol 16:695–701
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Hwang I, Ahn G, Park E, Ha D, Song JY, Jee Y (2011) An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol Lett 138:169–178
Hwang I, Ha D, Ahn G, Park E, Joo H, Jee Y (2011) Experimental autoimmune encephalomyelitis: association with mutual regulation of RelA (p65)/NF-kappaB and phospho-IkappaB in the CNS. Biochem Biophys Res Commun 411:464–470
Liu YM, Liu XJ, Bai SS, Mu LL, Kong QF, Sun B, Wang DD, Wang JH, Shu S, Wang GY, Li HL (2010) The effect of electroacupuncture on T cell responses in rats with experimental autoimmune encephalitis. J Neuroimmunol 220:25–33
Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM (2006) Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol 36:2139–2149
Le Y, Zhou Y, Iribarren P, Wang J (2004) Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol 1:95–104
Moser B, Loetscher P (2001) Lymphocyte traffic control by chemokines. Nat Immunol 2:123–128
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2007-0054931, 2010-0010858, and 2010-0026575). This study was also supported by the Traditional Korean Medicine R&D program founded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HI13C0263).
Conflict of Interest
All authors of this manuscript have no conflict of interest relevant to this subject.
Authors’ Contributions
MJL performed the behavioral experiment, immunohistochemistry, PCR analysis, flow cytometry analysis, Western blot analysis, and prepared the figures. JHC and MJ assisted in behavioral experiments. GL and HJM commented on the flow cytometry analysis. WSC, JIK, YC, and SHK commented on treatment with BV/BVA. SJL, YJ, YC, and SHK contributed to the interpretation of data and supervised the project. IHC conceived all experiments, analyzed the results, and wrote the manuscript. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Data 1
Photograph showing the location of the ST36 acupoint, non-acupoints, and neurological abnormalities after immunization (A-E) The location of the ST36 acupoint and six placebo-acupoints. ST36 (Zusanli) acupoint is located 5 mm below and lateral to the anterior tubercle of the tibia (A and B). SP9 acupoint is located in a depression between the posterior border of the tibia and the gastrocnemius muscle near the knee joint or the inferior border of the medial condyle of the tibia (A and C). GB39 is located distal 4/5 points on the imaginary line connecting the lateral side of the knee and the lateral malleolus of the tibiofibula (A and B). NA1 is localized on the proximal edge of the patella (A), NA2 is located on the lower leg, 3 units lateral to and below ST36 (A and B), NA3 is localized in the gluteal region near the ischial tuberosity (D), and NA4 is localized on the base of the proximal tail, approximately 3 cm from the posterior border of the gluteus muscle in rats (E). The NA1-4 location was chosen as it does not coincide with any classically defined acupoint or meridian structure, and it is located on the hindlimb and tail of rats and mice. A, anterior view; B and E, lateral view; C, medial view; D, ventral view (PDF 126 kb)
Supplementary Data 2
Photograph showing the neurological abnormalities after immunization. (A-H) The scale of neurological abnormalities after immunization in acute EAE rats. Grade 0, absence of symptoms (A); Grade 0.5, partial loss of tail tonus (B and C); Grade 1, paralysis of the tail (D); Grade 2, paraparesis of the hindlimb (E); Grade 3, paraplesis (F); Grade 4, paraplegia (G); Grade 5, tetraplegia (H); Grade 6, death. (I-Q) The scale of neurological abnormalities after immunization in chronic EAE mice. Grade 0, absence of symptoms (I and J); Grade 1, partial loss of tail tonus (K); Grade 2, paralysis of the tail (L and M); Grade 3, paraparesis (N); Grade 4, paraplegia (O); Grade 5, tetraparesis (P); Grade 6, tetraplegia (Q); Grade 7, death (PDF 231 kb)
Supplementary Data 3
Treatment with BVA at the placebo-acupoints away from ST36 fails to alleviate neurological impairment and histopathological changes in the spinal cord (A) BVA treatment into the placebo-acupoints [NA3 (on the gluteal region near the ischial tuberosity) and NA4 (on the base of the proximal tail located approximately 3 cm from the posterior border of the gluteus muscle) as nonspecific acupoints away from ST36] was performed every 3 days from 20 min before immunization. Rats were scored daily until 21 days. Treatment with BVA did not improve the neurological impairment after immunization. Data are expressed as mean clinical scores ± SEM. (ANOVA test; *p < 0.01 and **p < 0.05 versus MBP + saline-treated rats). (B-S) Spinal cord sections obtained from rats in each group (n = 3-5) at day 15–16 post-immunization were analyzed with respect to the degree of demyelination by luxol fast blue stain (B-J) and the degree of recruitment/infiltration of immune cells by H & E stain (K-S). Treatment with BVA at the placebo-acupoints did not block demyelination (B-J) and recruitment/infiltration of immune cells (K-S) in the spinal cord of rats acute EAE. Panels F-I and O-R display high magnification micrographs of panels B-E and K-N marked with squares, respectively. Bars =100 μm. (ANOVA test; #p < 0.01 and ##p < 0.05 versus normal rats) (PDF 339 kb)
Rights and permissions
About this article
Cite this article
Lee, M.J., Jang, M., Choi, J. et al. Bee Venom Acupuncture Alleviates Experimental Autoimmune Encephalomyelitis by Upregulating Regulatory T Cells and Suppressing Th1 and Th17 Responses. Mol Neurobiol 53, 1419–1445 (2016). https://doi.org/10.1007/s12035-014-9012-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9012-2